b College of Chemistry and Chemical Engineering, Fujian Normal University, Fuzhou 350007, China;
c State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China
As one of the most essential trace elements in biological systems,Fe3+ performs a major role in many biochemical processes at the cellular level [1]. The variation of Fe3+ concentrations within the cell will produce significant influences on physiological functions of cells,or even to the whole body [2]. Therefore,a convenient and rapid method for the analysis of Fe3+ in cellular systems has important consequences in biological and pathological concerns. Several analytical techniques have been adopted for the detection of Fe3+,among them,the method based on a fluorescent probe has received considerable interest in recent years because of its ability to provide a simple,sensitive,selective, precise and nondestructive examination for Fe3+ in cellular systems without any pre-treatment of the sample together with the advantages of spatial and temporal resolution [3].
Rhodamine dyes appear to be particularly attractive for the construction of an ‘‘off-on’’ type fluorescent probe,owing to their simplicity,low detection limit,the capability for special recognition and excellent spectroscopic properties [4]. A number of rhodamine-based fluorescence probes for Fe3+ with high sensitivity and selectivity have been developed,but many of them can only be used in non-aqueous media [5]. As a practical application of the probes in biological studies (such as cell imaging studies),good water solubility is essential,since the usage of organic solvents usually destroys the normal function of biomolecules [6]. As a result,there have been relatively few rhodamine-based fluorescent probes for Fe3+ which can be used directly in cells [7].
It is well known that siderophores are a kind of highly effective, water-soluble,iron-complexing ligands in plant cells and bacteria, by which plants and bacteria mobilize Fe3+ and deliver it to the cell [8]. More than 300 naturally occurring siderophores have been isolated and characterized,which all possess the same structural characteristics,i.e. multiple oxygen-containing haptos [9]. Synthetic siderophore analogs connected suitably to the rhodamine group is,therefore,a good strategy to build Fe3+-selective fluorescent probes. Surprisingly,so far as we know,only two probes based on such strategy have been reported [10]. Herein,we demonstrate a new biomimetic siderophore analog fluorescent probe for Fe3+ based on diglycolamine,a water-soluble multiple oxygen-containing compound,coupled with rhodamine B. We expect that the diglycolamine chain could be an iron-complexing subunit and give stable sensor-iron complexes in aqueous media due to the multiple oxygen-containing group.
2. ExperimentalThe 1H NMR and 13C NMR spectra were recorded on a Varian Unity-400 spectrometer operating at 400 MHz and 100 MHz, respectively,with tetramethylsilane as an internal reference. All chemical shifts are reported in the standard δ notation of parts per million. Mass spectrometric data were obtained on an AXIMACFRTM plus MALDI-TOF Mass Spectroscopy (Kratos Analytical,UK). UV-vis absorption spectra were recorded with a Shimadzu UV- 2400 spectrophotometer. All fluorescence measurements were conducted on a Hitachi F-7000 fluorescence spectrometer with excitation slit set at 5.0 nm and emission at 5.0 nm. Fluorescence images of Hela cells were carried out with an inverted fluorescence microscope (OLYMPUS BX43). All reagents were obtained commercially for synthesis and used without further purification. Pure water (18.2 Ω) was used to prepare all aqueous solutions.
The probe was synthesized based on modifications of the method previously described [11] using commercial reagents. The synthetic route of the probe is depicted in Scheme 1,and was fully characterized by 1H NMR,13C NMR,and MALDI-TOF MS (Figs. S1- S3 in Supporting information). Rhodamine B (479 mg,1 mmol), NaOH (80 mg,2 mmol) were dissolved in anhydrous ethanol (30 mL),and diglycolamine (2 mL,20 mmol) were added under N2 protection. The mixture was stirred under reflux conditions for 12 h under an N2 atmosphere. The solvent was removed under reduced pressure,then purified by aluminum oxide chromatography eluted with petroleum ether/ethyl acetate (1:1,v/v) to afford the desired product as a white solid (330 mg,yield 63%). Mp: 67- 69 8C,1H NMR (400 MHz,CDCl3): δ 7.97-7.88 (m,1 H),7.50-7.41 (m,2H),7.15-7.06 (m,1H),6.48-6.46 (d,2H,J = 8.8 Hz),6.39 (d, 2H,J = 2.4 Hz),6.31-6.28 (dd,2H,J1 = 8.8,J2 = 2.4 Hz),3.64-3.56 (t, 2H,J = 4.4 Hz),3.44-3.28 (m,14H),3.20 (t,2H,J = 6.4 Hz),1.19 (t, 12H,J = 7.2 Hz). 13C NMR (100 MHz,CDCl3): δ 168.54,153.61, 153.31,148.81,132.40,131.06,128.93,128.00,123.75,122.85, 108.09,105.59,97.72,77.39,77.07,76.75,72.01,68.36,65.04, 61.68,44.36,39.79,29.69,12.59. MALDI-TOF MS calcd. for C32H39N3O4 [M+H+]: 530.6778; found: 530.6744.

|
Download:
|
| Scheme 1. Synthetic pathway of the probe. | |
All the spectroscopic studies were performed in water/DMSO (99:1,v/v) in which the probe formed a colorless solution that was stable for more than three days. The solution of the probe was very weakly fluorescent in the absence of any analyte due to the predominant ring-closed spirolactone. The characteristic peak at δ = 65.04 ppm in the 13C NMR spectrum of the probe (Fig. S2) also supports this conclusion.
Since the rhodamine-based compounds are colored (emit fluorescent signal at the same time) in acidic solutions due to the proton-driven ring opening [12],the effect of pH was initially investigated. A pH-dependent absorbance profile was generated with a series of buffer solutions (Fig. 1). As shown in Fig. 1 the probe was non-absorpting within the pH range 4-10,and exhibited a considerable level of absorption only below pH 4.0. This observation suggested that the probe would be undisturbed by pH in biological samples.

|
Download:
|
| Fig. 1. Effect of pH on the absorbance of the probe (10 μmol/L) in water-DMSO (99:1,v/v). | |
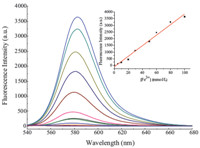
The fluorescence titration of the Fe3+ was carried out using a solution of 10 μmol/L of the probe in buffered (Tris-HCl,pH 7.3) water/DMSO (99:1,v/v) solution (Fig. 2). Upon addition of increasing concentrations of Fe3+,a remarkable enhancement in emission intensity was observed at 585 nm (Fig. 2) which was due to the ring-opening reaction producing the rhodamine spirolactam ring-opened form. As shown in the inset of Fig. 2,the plot of fluorescence intensity as a function of concentrations of Fe3+ is approximately linear in the range from 2 × 10-6 mol/L to 10-4 mol/L (R2 = 0.9863),with a detection limit of 3.5 × 10-7 mol/L (based on S/N = 3).
|
Download:
|
| Fig. 2. Fluorescence spectra of the probe (10 μmol/L) upon titration of Fe3+ (from bottom to top: 0,5,10,20,30,50,60,80,100 μmol/L) in buffered (Tris-HCl,pH 7.3) water/DMSO (99:1,v/v) solution (λex = 520 nm). Inset: plot of fluorescence intensity at 585 nm versus the number of equivalents of Fe3+. | |
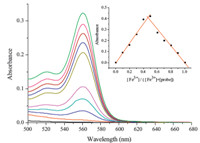
The changes in the UV-vis spectra with the addition of various concentrations of Fe3+ in buffered (Tris-HCl,pH 7.3) water/DMSO (99:1,v/v) solution were also investigated (Fig. 3). The colorless, free probe shows almost no absorption near 561 nm. Upon the gradual addition of Fe3+ to the solution of the probe,a new absorption peak at 561 nm emerged with increasing intensity. The solution displayed a distinct change from colorless to pink simultaneously,implying that the probe can serve as a visual or ‘‘naked eye’’ probe for detecting Fe3+. Job’s method for the absorbance was applied to determine the stoichiometry of the probe-Fe3+ complex by keeping the sum of the initial concentration of Fe3+ and the probe at 200 μmol/L and changing the molar ratio of Fe3+ from 0 to 1. As shown in the inset of Fig. 3,a maximum absorption was observed when the molar fraction of the [Fe3+] versus [Fe3+]+[probe] was 0.5,which indicated that a 1:1 complex was formed.
|
Download:
|
| Fig. 3. Absorption spectra of the probe (10 μmol/L) upon titration of Fe3+ (from bottom to top: 0,5,10,20,30,50,60,80,100 μmol/L) in buffered (Tris-HCl,pH 7.3) water/DMSO (99:1,v/v) solution. Inset: Job’s plot for the probe in the test systems. The total concentration of the probe and Fe3+ was 200 μmol/L. | |
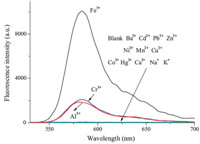
It is very important for a good probe to have high selectivity.We tested the probe for possible interferences,including nitrate salts of Ba2+,Cd2+,Pb2+,Zn2+,Ni2+,Mn2+,Cu2+,Co2+,Hg2+,Ca2+,Na+,K+, Cr3+ and Al3+ (Fig. 4.). Among the tested metal ions,Cr3+ and Al3+ showed a slight fluorescent enhancement,while the other tested metal ions showed only very weak responses. The anti-interference experiment also showed only slight interferences from Cr3+ and Al3+ (Fig. S4 in Supporting information). Considering the concentration of Cr3+ [13] or Al3+ [14] in normal human cells is very low,the Cr3+ and Al3+ is hardly competitive with Fe3+. It suggested that the probe showed an acceptable selectivity toward Fe3+ over other competitive cations.
|
Download:
|
| Fig. 4. Fluorescent spectra of the probe (10 μmol/L) in buffered (Tris-HCl,pH 7.3) water/DMSO (99:1,v/v) solution with the addition of 30 equiv different ions (λex = 520 nm). | |
The usefulness of the probe for fluorescence imaging of Fe3+ in living cells was also investigated. HeLa cells were cultured as the sample,and stained with the probe within 30 min and washed by PBS buffer,no fluorescence was detected (Fig. 5b). After further incubation at 37 8C with FeCl3 for another 30 min,a red fluorescence appeared in the intracellular region (Fig. 5e). Bright filed image of HeLa cells treated with the probe and Fe3+ confirmed that the cells were viable throughout the imaging experiments (Fig. 5d). An overlay of fluorescence and bright-field images shows that the fluorescence signals are localized in the intracellular area, indicating a subcellular distribution of Fe3+ and good cellmembrane permeability of the probe (Fig. 5f). These facts implied that the probe was membrane-permeable and could sense intracellular Fe3+ in living cells.

|
Download:
|
| Fig. 5. Images of HeLa cells treated with the probe: (a) bright field image of HeLa cells incubated with the probe (10 μmol/L); (b) fluorescence image of HeLa cells incubated with the probe (10 μmol/L); (c) the overlay image of (a) and (b); (d) bright field image of HeLa cells incubated with the probe (10 μmol/L) for 30 min,and then further incubation at 37 ℃ with Fe3+ (20 μmol/L) for 30 min; (e) fluorescence image of (d) from red channel; (f) the overlay image of (d) and (e). | |
According to the Job’s plot for the probe (Fig. 3. Inset) and the ref [10],a possible interaction mode of probe-Fe3+ was Fe3+ chelating with carbonyl oxygen,oxygen and nitrogen of diglycolamine while the other coordination sites were occupied by solvent molecules (Scheme 2).

|
Download:
|
| Scheme 2. Proposed mechanism of Fe3+ -induced opening of the probe ring. | |
In summary,we have developed a simple rhodamine-based fluorescent probe which exhibited a ‘‘turn-on’’ fluorescent and colormetric signal toward Fe3+ with an acceptable selectivity in aqueous solution containing 1% DMSO. The probe can be used to detect Fe3+ over such a wide pH range that the probe would be undisturbed by pH in biological samples. Fluorescence microscopy imaging experiments have proven that the probe is cell permeable and can be used for monitoring intracellular Fe3+ in living cells.
AcknowledgmentThisworkwas supportedbyNatural Science Foundationof Fujian Province (No. 2013H0019),the Science Foundation of Education Department of Fujian Province (No. JA11064),the Open Foundation of the State Key Laboratory of Fine Chemicals (No. KF1307),and the Open Foundation of Ministry of Education Key Laboratory of Synthetic and Natural Functional Molecular Chemistry.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.09.025.
| [1] | (a) B. William, S. Maya, Intracellular labile iron, Int. J. Biochem. Cell Biol. 40 (2008) 350-354;(b) C.D. Kaplan, J. Kaplan, Iron acquisition and transcriptional regulation, Chem. Rev. 109 (2009) 4536-4552. |
| [2] | (a) B. Halliwell, J.M.C. Gutteridge, Free Radicals in Biology and Medicine, Oxford University Press, Oxford, 1999, pp. 55-56;(b) P. Frank, P. Sandra, E. Dogruöz, et al., Reduction of Fe(Ⅲ) ions complexed to physiological ligands by lipoyl dehydrogenase and other flavoenzymes in vitro, J. Biol. Chem. 278 (2003) 46403-46413;(c) C.H. Robert, X.L. Kong, Iron speciation in the cytosol: an overview, Dalton Trans. 42 (2013) 3220-3229;(d) P. Wang, T.A. Okamura, H.P. Zhou, W.Y. Sun, Y.P. Tian, Metal complex with terpyrindine derivative ligand as highly selective colorimetric sensor for iron(Ⅲ), Chin. Chem. Lett. 24 (2013) 20-22. |
| [3] | (a) K.M. Dean, Y. Qin, A.E. Palmer, Visualizing metal ions in cells: an overview of analytical techniques, approaches, and probes, Biochim. Biophys. Acta 1823 (2012) 1406-1415;(b) C. Giselle, M.M. Tania, R.B. Fernanda, Analytical methods for copper, zinc and iron quantification in mammalian cells, Metallomics 5 (2013) 1336-1345. |
| [4] | (a) H.N. Kim, M.H. Lee, H.J. Kim, J.S. Kim, J. Yoon, A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions, Chem. Soc. Rev. 37 (2008) 1465-1472;(b) X.Q. Chen, T.H. Pradhan, F. Wang, J.S. Kim, J. Yoon, Fluorescent chemosensors based on spiroring-opening of xanthenes and related derivatives, Chem. Rev. 112 (2012) 1910-1956. |
| [5] | (a) X. Zhang, Y. Shiraishi, T. Hirai, A new rhodamine derivative bearing an azacrown ether as a selective fluorescent chemosensor for Fe3+ and Hg2+, Tetrahedron Lett. 49 (2008) 4178-4181;(b) L.Z. Zhang, J.L. Fan, X.J. Peng, X-ray crystallographic and photophysical properties of rhodamine-based chemosensor for Fe3+, Spectrochim. Acta Part A 73 (2009) 398-402;(c) T.L. Gao, K.M. Lee, J.Y. Heo, S.I. Yang, A new ferric ion-selective fluorescent chemosensor with a wide dynamic range, Bull. Korean Chem. Soc. 31 (2010) 2100-2102;(d) J.B. Li, Q.H. Hu, X.L. Yu, et al., A novel rhodamine-benzimidazole conjugate as a highly selective turn-on fluorescent probe for Fe3+, J. Fluoresc. 21 (2011) 2005- 2013;(e) W.T. Yin, H. Cui, Z. Yang, et al., Facile synthesis and characterization of rhodamine-based colorimetric and "off-on" fluorescent chemosensor for Fe3+, Sens. Actuators B 157 (2011) 675-680;(f) M.Y. She, Z. Yang, B. Yin, et al., A novel rhodamine-based fluorescent and colorimetric"off-on" chemosensor and investigation of the recognizing behavior towards Fe3+, Dyes Pigments 92 (2012) 1337-1343;(g) Z. Aydin, Y.B. Wei, M.L. Guo, A highly selective rhodamine based turn-on optical sensor for Fe3+, Inorg. Chem. Commun. 20 (2012) 93-96. |
| [6] | (a) J.J. Du, M.M. Hu, J.L. Fan, X.J. Peng, Fluorescent chemodosimeters using "mild" chemical events for the detection of small anions and cations in biological and environmental media, Chem. Soc. Rev. 41 (2012) 4511-4535;(b) M.H. Lynne, J.F. Katherine, Probing oxidative stress: small molecule fluorescent sensors of metal ions, reactive oxygen species, and thiols, Coord. Chem. Rev. 256 (2012) 2333-2356;(c) X.H. Li, X.H. Gao, W. Shi, H.M. Ma, Design strategies for water-soluble small molecular chromogenic and fluorogenic probes, Chem. Rev. 114 (2014) 590-659. |
| [7] | (a) S.R. Liu, S.P. Wu, New water-soluble highly selective fluorescent chemosensor for Fe(Ⅲ) ions and its application to living cell imaging, Sens. Actuators B 171-172 (2012) 1110-1116;(b) H.J. Sheng, X.M. Meng, W.P. Ye, et al., A water-soluble fluorescent probe for Fe(Ⅲ): improved selectivity over Cr(Ⅲ), Sens. Actuators B 195 (2014) 534-539;(c) C.Y. Li, C.X. Zou, Y.F. Li, J.L. Tang, C. Weng, A new rhodamine-based fluorescent chemosensor for Fe3+ and its application in living cell imaging, Dyes Pigments 104 (2014) 110-115;(d) Z. Yang, M.Y. She, B. Yin, et al., Three rhodamine-based "off-on" chemosensors with high selectivity and sensitivity for Fe3+ imaging in living cells, J. Org. Chem. 77 (2012) 1143-1147;(f) M.P. Yang, C.C. Xu, S.N. Li, et al., Three selective and sensitive "off-on" probes based on rhodamine for Fe3+ imaging in living cells, RSC Adv. 4 (2014) 14248- 14253. |
| [8] | K.N. Raymond, Biomimetic metal encapsulation, Coord. Chem. Rev. 105 (1990) 135-155. |
| [9] | S.K. Sahoo, D. Sharma, R.K. Bera, G. Crisponi, J.F. Callan, Iron(Ⅲ) selective molecular and supramolecular fluorescent probes, Chem. Soc. Rev. 41 (2012) 7195-7227. |
| [10] | (a) S. Bae, J. Tae, Rhodamine-hydroxamate-based fluorescent chemosensor for FeⅢ, Tetrahedron Lett. 48 (2007) 5389-5392;(b) K.S. Moon, Y.K. Yang, S. Ji, J. Tae, Aminoxy-linked rhodamine hydroxamate as fluorescent chemosensor for Fe3+ in aqueous media, Tetrahedron Lett. 51 (2010) 3290-3293. |
| [11] | (a) Y. Shiraishi, R. Miyamoto, X. Zhang, T. Hirai, Rhodamine-based fluorescent thermometer exhibiting selective emission enhancement at a specific temperature range, Org. Lett. 9 (2007) 3921-3924;(b) M.H. Lee, H.J. Kim, S. Yoon, N. Park, J.S. Kim, Metal ion induced FRET off-on in tren/dansyl-appended rhodamine, Org. Lett. 10 (2008) 213-216. |
| [12] | J.D. Chartres, M. Busby, M.J. Riley, J.J. Davis, P.V. Bernhardt, A turn-on fluorescent iron complex and its cellular uptake, Inorg. Chem. 50 (2011) 9178-9183. |
| [13] | A.K. Singh, V.K. Gupta, B. Gupta, Chromium(Ⅲ) selective membrane sensors based on Schiff bases as chelating ionophores, Anal. Chim. Acta 585 (2007) 171-178. |
| [14] | R. Patil, A. Moirangthem, R. Butcher, et al., Al3+ selective colorimetric and fluorescent red shifting chemosensor: application in living cell imaging, Dalton Trans. 43 (2014) 2895-2899. |




