b College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, China;
c School of Chemistry and Environment, Beijing University of Aeronautics & Astronautics, Beijing 100191, China
Due to its high specific capacity and low Li intercalation potential,SnO2 is considered as one of the most promising next generation anode material candidates for Li ion batteries [1- 17]. SnO2 has a prior step of reductive reaction before reversible process and can be written as follows:
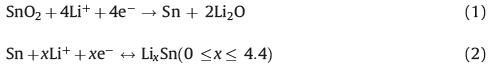
Generally,the reaction in Eq. (1) is irreversible or only partially reversible and the theoretical capacity of SnO2 (782 mAh/g) is mainly caused by the reaction in Eq. (2) [1, 2]. The high capacity seems to have an advantage relative to conventional graphite (372 mAh/g). However,the reaction process accompanied with large volume variation (may attain 259%) [3]. The reactive material may undo continuously large volume expansion or shrink during Li uptake or release,which could lead to pulverization. Only solving this problem,the performance of SnO2 anode material can be really enhanced.
To overcome these problems,many strategies have been developed during the past decade and they can be divided into two categories. The first one is to prepare composite electrodes with conductive materials,such as carbon [4],carbon nanotubes [5, 6, 7],and graphene [8, 9]. The above mentioned conductive matrix used not only acts as a physical buffering layer for the large volume change but also as a conductor that enhances the electronic conductivity of the electrode. The second one is to prepare nanomaterials with unique morphologies [10, 11, 12, 13]. The nanostructured materials open up a new important avenue in the advancement of the science and technology of LIB applications. Nanomaterials bring us the advantages of higher electrode/ electrolyte contact area,short path lengths for Li+ transport,high power performance and new reactions,which are not possible with bulk materials.
Recently,it is reported that SnO2 hollow nanospheres have displayed improved cycling performance,as the hollow interiors can effectively buffer the volume change of the anode [1,14- 18]. Although limited success has been achieved,attempts to further improve the electrochemical performance of SnO2 anode is still a challenge to scientists. Taking the SnO2 hollow spheres as example,we hope the void volume large and effective. However, on one hand,when the shell of the sphere is too thick (the central cavity is too small),the cycling performance would not be satisfied because the limited buffer space can not sufficiently alleviate the volume change of the anode [17]; on the other hand,when the shell of the sphere is too thin (the central cavity is too large),generally, the bare sphere would become fragile and the durability of the anode would still not be satisfied [18]. In our previous study,we found that a of SnO2 nanocubes with suitable pore volume exhibit best cycling performance as anode material for Li ion battery [19]. Therefore,we speculated that,only a hollow sphere with suitable shell thickness/diameter ratio,in other words,with appropriate pore volume [19],can provide the stable structural stability and the excellent cycling performance of the SnO2 anode.
Herein,we report a facile one-pot solvothermally synthesized SnO2 hollow nanospheres with ultra thin shell (~10 nm) and diameter of ~50 nm. The suitable shell/diameter ratio and large surface area of the material promise the material with excellent electrochemical performance.
2. Experimental 2.1. Synthesis of materialsAll chemicals used in this experiment were analytical grade and were used without further purification. In a typical experiment, 1 mmol anhydrous Na2SnO3 (Aladdin) was dissolved in the solution containing 10 mL ethylene glycol and 30 mL DI water. After 30 min of magnetic stirring,a transparent solution was obtained. The solution was transferred into a 50 mL Teflon-lined autoclave. Then the autoclave was sealed and heated to 150 ℃ and maintained for 24 h. After cooling down to room temperature, white precipitate was obtained after washed with DI water and alcohol for several times.
2.2. CharacterizationsThe samples were characterized by X-ray powder diffraction (XRD) with a Rigaku Dmax 2200 X-ray diffractometer equipped with Cu-Kα radiation (λ = 1.5416Å),recorded with the 2u ranging from 20° to 80°. The morphology of the samples was characterized using scanning electron microscopy (SEM) (Apollo 300). The detailed microstructure analysis,containing TEM,HRTEM and SAED,was carried out by transmission electron microscopy (TEM) (JEOL JEM-2100F). Specific surface areas and void volumes of the samples were measured at 77 K by Brunauer-Emmett-Teller (BET) nitrogen adsorption-desorption (NOVA 2200e,Quanthachrome, USA).
2.3. Electrochemical performanceThe electrochemical reactions of samples with lithium were investigated using a simple two-electrode cell. Electrode was made in the conventional way: a slurry was first obtained by thoroughly mixing 80 wt% of the active materials,10 wt% of carbon black,and 10 wt% polyvinylidene fluoride (PVDF) in N-methyl-2-pyrrolidone (NMP) solvent. The slurry was then cast on a copper disk of 14 mm in diameter and further dried at 80 ℃ for 10 h. Before used,the film electrode was pressed at 10 MPa. The 2016 coin cells were assembled in an Ar-filled glove box,using microporous polypropylene (PP) as the separator,a solution of 1 mol/L LiPF6 in ethylene carbonate (EC) and dimethyl carbonate (DMC) (1:1 by volume) as electrolyte and a Li-metal circular foil as counter and reference electrode. The charged and discharged tests were performed galvanostatically on a CT2001A Land battery testing system. The cells were charged and discharged at various current and a constant temperature of 25 ℃ in the voltage ranges of 0.005-1.5 V. The electrochemical impedance measurements were performed on CHI660D electrochemical workstation (Shanghai Chenhua Co.,Ltd., China) at an AC voltage of 5 mV amplitude in the 100 kHz to 0.01 Hz immediately the SnO2 sample electrodes run over 20 cycles.
3. Results and discussionsA typical XRD pattern of the as-prepared SnO2 sample is shown in Fig. 1 to characterize the crystal structure of the product. Indices of crystallographic planes have been marked. All of the diffraction peaks are in accordance with the standard bulk SnO2 pattern (JCPDS: 41-1445) and can be well indexed to the tetragonal rutile phase SnO2. No peaks of impurity signals were detected,indicating the high purity of the product. Based on the Scherrer equation, which can be described as D = (0.89l)/b(cosu),in which λ is the wavelength for the Kα1(1.5406Å),β is the peak width at halfmaximum in radians,and θ is the Bragg angle,we can calculate the average particle size is 10.9 nm.

|
Download:
|
| Fig. 1. Room-temperature XRD pattern of the as-prepared SnO2 product. | |
Fig. 2 shows the overview morphology of as-prepared SnO2 product that was studied by SEM. A number of uniform spheres can be seen in Fig. 2(a). From the Fig. 2(b),it can be found that these spheres are about 50 nm in diameter. The surface of the spheres is very rough,which implies the polycrystalline nature of the material. To better display the morphology and crystal structure of the spheres,TEM,HRTEM accompanied with selected area electron diffraction (SAED) was performed,as depicted in Fig. 3. Fig. 3(a) shows the TEM image of the SnO2 nanospheres. We can see clearly the spheres have homogeneous structure and particle size. The diameter of the spheres is about 50 nm,which matches well the SEM observations. The obvious light and dark contrast between the edge and the center of the spheres implies the hollow interior of the SnO2 nanospheres. Similar to the previously reports [1, 16, 17, 20, 21],the formation of hollow structure may be relative to the action of ethylene glycol. Compared with ethanol,the higher viscosity of ethylene glycol may slow down the diffusion rate of precursor ions in the mixture solution,leading to the formation of much smaller SnO2 constructions. Fig. 3(b) is the magnified TEM image of a SnO2 sphere. As can be seen from Fig. 3(b),the primary SnO2 nanoparticles that assembled the sphere have a diameter of ~10 nm. Comparatively,the shell of the SnO2 nanosphere is approximate 10 nm in thickness. It means that,the shell is assembled by one layer SnO2 particles. The unique structural feature of the material promises it with large surface area,which will be introduced in the following section. The high-resolution TEM (HRTEM) image,as shown in Fig. 3(c),depicts clear lattice fringes of small SnO2 nanocrystals. The interplanar spacing are measured to be 0.33 nm and 0.26 nm,which are consistent with the d values of the (1 1 0) and (1 0 1) planes of the tetragonal rutile SnO2,respectively. The diffraction rings in the SAED pattern (Fig. 3(d)) clearly reveal the polycrystalline nature of the SnO2 hollow spheres. From inside to outside,they can be assigned to (1 1 0),(1 0 1),(2 0 0),(2 1 1) and (3 0 1) planes. From the above information,we can call the SnO2 hollow spheres as ‘‘HS-SnO2’’.

|
Download:
|
| Fig. 2. (a) Low and (b) high-magnification SEM images of the as-prepared SnO2 nanospheres. | |

|
Download:
|
| Fig. 3. (a) Low and (b) high-magnification TEM images,(c) HRTEM image of SnO2 hollow spheres. (d) SAED pattern for the SnO2 product. | |
The specific surface area and the pore size distribution of the HS-SnO2 are further characterized by nitrogen adsorption and desorption isotherms at 77 K,as shown in Fig. 4. The isotherm is characteristic of a type IV with a type H3 hysteresis loop,revealing the presence of mesopores in the size range 2-20 nm(Fig. 4(inset)). The BET surface area of the sample was measured to be 202.5 m2/g. The pore volume of the sample was measured to be and 0.251 cc/g, which is close to the values (0.257 cc/g,0.244 cc/g) of the SnO2 nanocubes we reported before [19]. As the large surface area of the HS-SnO2 can provide more reaction sites with Li and the hollow inter structure of the material can also release the volume change during the Li insertion and de-insertion process,excellent cycling performance of the HS-SnO2 anode can be expected.

|
Download:
|
| Fig. 4. Nitrogen adsorption/desorption isotherms and the corresponding pore size distributions (inset) of the as prepared product. | |
In order to testify the superior electrochemical performance of the HS-SnO2,commercial SnO2 particles were employed for comparison. Fig. 5 shows the first discharge-charge curves of the HS-SnO2 and the commercial SnO2 particles. As shown in Fig. 5, at the current of 0.5 (375 mAh/g),the initial discharge/charge capacities corresponding to the HS-SnO2 are 1597 mAh/g and 827 mAh/g,respectively. And the initial discharge/charge capacities of the commercial SnO2 particles are 1394 mAh/g and 495 mAh/g,respectively. The HS-SnO2 exhibited much higher discharge/charge capacities than the SnO2 particles. Furthermore, the initial coulombic efficiency of each sample is 51.7% and 35.5%, respectively. The HS-SnO2 also showed higher initial coulombic efficiency than the SnO2 particles.

|
Download:
|
| Fig. 5. Discharge/charge curves of the HS-SnO2 and the commercial SnO2 at 0.5 C. | |
Fig. 6a compares the cycling performance of both samples tested under the same condition. As expected,after 20 cycles the discharge/charge capacities of HS-SnO2 are 709 mAh/g and 695 mAh/g,respectively,a high reversible (charge) capacity retention up to 84% can be obtained. While the SnO2 particles only show a discharge/charge capacities of 161 mAh/g and 155 mAh/g after 20 cycles with reversible capacity retention of 31%.

|
Download:
|
| Fig. 6. (a) Cycling performance of the HS-SnO2 and the commercial SnO2; (b) cycling performance of HS-SnO2 at varying current rate. | |
To fully evaluate the electrochemical performance of the HSSnO2, the electrode was firstly cycled at 0.5 C for 20 cycles,then adjusted to 1 C for another 20 cycles,and finally adjusted to 0.5 C for 20 cycles. The cyclic data are shown in Fig. 6(b). As can be seen from Fig. 6(b),the cycling data of the first 20 cycles is similar to Fig. 6(a),which shows a good capacity retention at low rate. At 1 C rate,discharge/charge capacities of 538 mAh/g and 505 mAh/g can still be reached,with neglectable capacity fading even after 20 cycles. When the current density was adjusted to 0.5 C,a reversible capacity can recover to a high point at 610 mAh/g,and even after 20 cycles,a capacity as high as 509 mAh/g can be obtained,which is still higher than the theoretical capacity of graphite (380 mAh/g). We speculate that,it is the suitable shell/ diameter ratio of the HS-SnO2 that promises the good structural stability of the material during cycling. In order to testify our speculation,the tested electrode was disassembled and observed by TEM. As shown in Fig. 7,after 60 cycles,a majority of the HSSnO2 still maintain their original morphology although some of them collapsed.

|
Download:
|
| Fig. 7. TEM images of the HS-SnO2 after 60 cycles. | |
Fig. 8 shows AC impedance spectra of the sample electrodes measured at the open potential of 1.5 V after 20 cycles. Both of them can be analyzed with the same equivalent circuit mode (the inset of Fig. 8). Rs,Rct,Zw,and CPE represent resistance of the electrolytes,charge-transfer resistance,Warburg resistance,and constant phase element. Generally,the semicircle of the AC impedance spectra is assigned to the charge-transfer impedance on electrode (Rct),and the inclined line in low-frequency corresponds to the lithium-diffusion process within electrodes (Rs) [22, 23]. From Fig. 8,it is clearly shown that the diameter of the semicircle for the HS-SnO2 spheres electrode is much smaller than that of commercial SnO2 electrode,which indicates that the product possess lower charge-transfer resistances when the surface area of both samples is same in the measurement of the impedance. The reduction of the resistance can be attributed to the more convenient ion diffusion routes. Both the smaller size and the single layer structure of the HS-SnO2 make contributions to the result. Indeed,as previously mentioned,the improved cycling performance can be considered for this reason in a large extent.

|
Download:
|
| Fig. 8. AC impedance spectra of the sample electrodes measured at the open potential of 1.5 V (after the 20th cycle). The inset left above is shown an equivalent circuit mode plot. | |
In summary,the remarkably improved electrochemical performance of the HS-SnO2 compared with commercial SnO2 particles, takes a great advantages of its unique structure. Firstly,the large surface area and the ultra thin shell of the HS-SnO2 can not only provide large electrode/electrolyte contact area but also shorten the Li ion diffusion path way,which lead to higher initial reversible capacity,coulombic efficiency and rate performance of the material. Secondly,the hollow interior provides enough interior space to buffer the large volume changes during Li insertion and de-insertion (taken and up taken) process; thirdly,the suitable shell/diameter ratio of the HS-SnO2 promises the good structure stability of the material and can undergo long cycling.
4. ConclusionsSingle layer nanocrystals assembled SnO2 hollow nanospheres were prepared via a facile solvothermal method. As Li ion battery anode material,the as prepared material showed excellent cycling performance and high rate property. The improved electrochemical property of this material closely related to its unique morphology and microstructure. The suitable shell/diameter ratio, the hollow interior and the ultra thin shell of the SnO2 hollow nanosphere promises electrochemical property of the material as Li ion battery anode material.
AcknowledgmentsThis work was financially supported by the National Basic Research Program of China (Nos. 2010CB934700,2013CB934004, 2011CB935704) and National Natural Science Foundation of China (No. 11079002).
| [1] | J.S. Chen, L. Archer, X.W. Lou, SnO2 hollow structures and TiO2 nanosheets for lithium-ion batteries, J. Mater. Chem. 21 (2011) 9912-9924. |
| [2] | X.W. Lou, J.S. Chen, P. Chen, L.A. Archer, One-pot synthesis of carbon-coated SnO2 nanocolloids with improved reversible lithium storage properties, Chem. Mater. 21 (2009) 2868-2874. |
| [3] | P. Meduri, E. Clark, E. Dayalan, G.U. Sumanasekera, M.K. Sunkara, Kinetically limited de-lithiation behavior of nanoscale tin-covered tin oxide nanowires, Energy Environ. Sci. 4 (2011) 1695-1699. |
| [4] | Y.M. Li, J.H. Li, Carbon-coated macroporous Sn2P2O7 as anode materials for Li-ion battery, J. Phys. Chem. C 112 (2008) 14216-14219. |
| [5] | N.H. Zhao, L.C. Yang, P. Zhang, et al., Polycrystalline SnO2 nanowires coated with amorphous carbon nanotube as anode material for lithium ion batteries, Mater. Lett. 64 (2010) 972-975. |
| [6] | Z.H. Wen, Q. Wang, Q. Zhang, J. Li, In situ growth of mesoporous SnO2 on multiwalled carbon nanotubes: a novel composite with porous-tube structure as anode for lithium batteries, Adv. Funct. Mater. 17 (2007) 2772-2778. |
| [7] | N.H. Zhao, G.J. Wang, Y. Huang, et al., Preparation of nanowire arrays of amorphous carbon nanotube-coated single crystal SnO2, Chem. Mater. 20 (2008) 2612- 2614. |
| [8] | Y.M. Li, X.J. Lü, J. Lu, J.H. Li, Preparation of SnO2-nanocrystal/graphene-nanosheets composites and their lithium storage ability, J. Phys. Chem. C 114 (2010) 21770- 21774. |
| [9] | S.J. Ding, D.Y. Luan, F. Boey, et al., SnO2 nanosheets grown on graphene sheets with enhanced lithium storage properties, Chem. Commun. 47 (2011) 7155-7157. |
| [10] | Q. Wang, Z.H. Wen, J.H. Li, Fast and reversible lithium-induced electrochemical alloying in tin-based composite oxide hierarchical microspheres assembled by nanoplate building blocks, J. Power Sources 182 (2008) 334-339. |
| [11] | H. Wang, Q.Q. Liang, W.J. Wang, et al., Preparation of flower-like SnO2 nanostructures and their applications in gas-sensing and lithium storage, Cryst. Growth Des. 11 (2011) 2942-2947. |
| [12] | F. Wang, S. Xiao, Z. Chang, Y.Q. Yang, Y.P. Wu, Nanoporous LiNi1/3Co1/3Mn1/3O2 as an ultra-fast charge cathode material for aqueous rechargeable lithium batteries, Chem. Commun. 49 (2013) 9209-9211. |
| [13] | W. Tang, Y.Y. Hou, F.X. Wang, et al., LiMn2O4 nanotube as cathode material of second-level charge capability for aqueous rechargeable batteries, Nano Lett. 13 (2013) 2036-2040. |
| [14] | X.M. Yin, C.C. Li, M. Zhang, et al., One-step synthesis of hierarchical SnO2 hollow nanostructures via self-assembly for high power lithium ion batteries, J. Phys. Chem. C 114 (2010) 8084-8088. |
| [15] | H.X. Yang, J.F. Qian, Z.X. Chen, X.P. Ai, Y.L. Cao, Multilayered nanocrystalline SnO2 hollow microspheres synthesized by chemically induced self-assembly in the hydrothermal environment, J. Phys. Chem. C 111 (2007) 14067-14071. |
| [16] | D. Deng, J. Lee, Hollow core-shell mesospheres of crystalline SnO2 nanoparticle aggregates for high capacity Li+ ion storage, Chem. Mater. 20 (2008) 1841-1846. |
| [17] | X.W. Lou,D.Deng, J.Y. Lee, L.A.Archer, PreparationofSnO2/carboncomposite hollow spheres and their lithium storage properties, Chem. Mater. 20 (2008) 6562-6566. |
| [18] | S.J. Ding, J.S. Chen, G.G. Qi, et al., Formation of SnO2 hollow nanospheres inside mesoporous silica nanoreactors, J. Am. Chem. Soc. 133 (2011) 21-23. |
| [19] | W. Wei, S. Gao, Z. Yang, et al., Porous SnO2 nanocubes with controllable pore volume and their Li storage performance, RSC Adv. 4 (2014) 13250-13255. |
| [20] | Z.W. Deng, M. Chen, G.X. Gu, L. Wu, A facile method to fabricate ZnO hollow spheres and their photocatalytic property, J. Phys. Chem. B 112 (2008) 16-22. |
| [21] | H.J. Zhang, J. Wu, L.P. Zhou, D.Y. Zhang, L.M. Qi, Facile synthesis of monodisperse microspheres and gigantic hollow shells of mesoporous silica in mixed water- ethanol solvents, Langmuir 23 (2007) 1107-1113. |
| [22] | C. Li, W. Wei, S.M. Fang, et al., A novel CuO-nanotube/SnO2 composite as the anode material for lithium ion batteries, J. Power Sources 195 (2010) 2939-2944. |
| [23] | J.F. Liang, W. Wei, D. Zhong, et al., One-step in situ synthesis of SnO2/graphene nanocomposites and its application as an anode material for Li-ion batteries, ACS Appl. Mater. Interfaces 4 (2012) 454-459. |




