b State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China
The genus Euphorbia is the largest in the spurge family, comprising of more than 2000 species,many of which have been used in folk as traditional Chinese medicine for the treatment of skin diseases,edemas,etc. [1]. Previous investigations on this genus revealed that the major bioactive constituents are diterpenoids [2, 3, 4, 5]. Euphorbia dracunculoides Lam.,as an annual or shortlived perennial herb,is distributed in riverbanks,valleys and roadsides of sandy areas in North Africa,South Europe,and Southwest Asia [6],and has been used as a folk medicine in India for its purgative and diuretic effects [7]. The earlier phytochemical investigations on E. dracunculoides are limited to the presence of flavonoids [7, 8, 9],triterpenoids [9],and coumarins [10]. To the best of our knowledge,there are no reports about diterpenoids from E. dracunculoides over the last two decades. In our efforts to search for structurally interesting and potential bioactive diterpenoids from genus Euphorbia,two new myrinsol diterpenoids euphordracunculins A (1) and B (2),together with three known analogues, euphorprolitherin B (3) [11],proliferins A (4) and B (5) [12],were isolated from the aerial parts of E. dracunculoides. In this paper,the isolation and structural elucidation of two new compounds are presented (Fig. 1).

|
Download:
|
| Fig. 1. Structures of compounds 1-5. | |
The aerial parts of E. dracunculoides Lam. were collected in September 2012 from Xishuang Banna prefecture,Yunnan Province,People’s Republic of China,and identified by Prof. Yao- Wen Yang,Yunnan University of Traditional Chinese Medicine. A voucher specimen (YTCM 20120915) was deposited at Yunnan University of Traditional Chinese Medicine. 2.2. Extraction and isolation
The air-dried and powdered aerial parts of E. dracunculoides Lam. (4.0 kg) were extracted with 70% aqueous acetone (8 L × 2 d × 3) at room temperature. The extracts were concentrated by a rotary evaporator under reduced pressure to remove organic solvent. The aqueous residue was then partitioned with petroleum ether (4 × 1 L),EtOAc (4 × 1 L),and n-BuOH (4 × 1 L) sequentially. The petroleum ether extract (48.0 g) was subjected to column chromatography (CC) on silica gel (200-300 mesh) using a gradient system of increasing polarity with petroleum ether- EtOAc (50:1,20:1,10:1,5:1,2:1,1:1,0:1) to afford six fractions (A- F) based on TLC analysis.
Fraction D (7.2 g) was decolorized on a MCI gel (CHP 20P) CC eluted by MeOH,and then divided into three subfractions (Fr. D-1- Fr. D-3) by silica gel (200-300 mesh) CC eluting with petroleum ether/Me2CO (15:1,6:1,1:1,respectively). Fr. D-1 was separated by Sephadex LH-20 column (MeOH/CHCl3,1:1),followed by semipreparative HPLC (HITACHI HPLC system; YMC-Triart C18 column,250 mm × 10 mm; DAD detector,MeOH/H2O 75:25, 232 nm,3.5 mL/min) to give compounds 3 (18.6 mg,tR = 25.8 min), 4 (9.0 mg,tR = 37.1 min),and 5 (3.2 mg,tR = 40.1 min). Fr. D-2 was chromatographed on Sephadex LH-20 column (MeOH),followed by preparative HPLC (CXTH LC-3000 HPLC system,Kromasil C18 column,250 mm × 20 mm; UV detector,MeOH-H2O 76:24, 232 nm,12.0 mL/min) to yield compound 2 (14.5 mg, tR = 13.8 min). Fr. D-3 was subjected to Sephadex LH-20 CC eluted by MeOH,followed by semipreparative HPLC (HITACHI HPLC system; YMC-Triart C18 column,250 mm × 10 mm; DAD detector, MeOH-H2O 77:23,232 nm,3.5 mL/min) to afford compound 1 (8.6 mg,tR = 15.9 min).
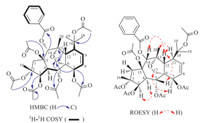
3. Results and discussionCompound 1,[α]D26:5 -134.0 (c 0.21,MeOH),UV(MeOH) λmax (log e): 271 (2.98),230 (4.08) and 201 (4.07) nm,obtained as colorless needles from MeOH,mp 197-200 ℃. Its molecular formula was determined to be C39H48O15 based on the HR-ESI-MS data (m/z 779.2887 [M+Na]+,calcd. 779.2891),corresponding to 16 degrees of unsaturation. Its IR spectrumshowed absorption bands for carbonyl groups at 1742 cm-1. The 1H NMR spectrum (Table 1) showed 10 3H-singlets at δH 2.15,2.10,2.06,2.00,1.98,1.71,1.64,1.55,1.33 and 1.22,of which six may be assigned for acetate groups and four be assigned for tertiary methyl groups. A mono-substituted benzene ring [δH 8.08 (d,2H,J = 7.8 Hz),7.58 (t,1H,J = 7.8 Hz),7.45 (t,2H, J = 7.8 Hz)] was also evident in the 1H NMR spectrum. Additionally, the signals of two vicinal olefinic protons [δH 6.18 (ddd,1H,J = 9.9, 6.6,1.5 Hz),5.91 (dd,1H,J = 9.9,5.6 Hz)] and an oxygenated methylene group [δH 4.16 (d,1H,J = 8.7 Hz),3.53 (dd,1H,J = 8.7, 1.6 Hz)] were also observed. Seven carbonyl signals at δC 170.9, 170.7,170.6,169.6,169.5,168.7 and 165.9,were obvious in the 13C NMR spectrum of 1. Accordingly,1 was presumably a highly oxidized tetracyclic diterpenoid substituted by six acetoxy and one benzoyloxy groups. Four oxymethine protons geminal to ester functions [δH 5.95 (dd,1H,J = 11.1,1.5 Hz),5.81 (1H,s),5.36 (br d, 1H,J = 4.1 Hz),4.86 (d,1H,J = 6.6 Hz)] suggested that the other three ester groups were located at quaternary carbons. Comparison the above NMR data with those of euphorprolitherin B (3),a myrsinol diterpene [11] which was also isolated in our present study, indicated that they are quite structurally similar. The possible differencewas that a propionyloxy groupatC-3 in euphorprolitherin B is replaced by an acetoxy group in 1,which was supported by the disappearance of theNMR signals of propionyloxyl and presence of a typical acetoxyl signals (δC 170.7,s,21.4,q; δH 2.06,s) in 1. The hypothesis was further verified by the HMBC correlations (Fig. 2) from a methyl signal at δH 2.06 (s,3H) and an oxymethine proton signal at δH 5.36 (br d,1H,J = 4.1 Hz,H-3) to an ester carbonyl signal at δC 170.7,respectively. The accurate assignments of all protons and carbons were preformed through the correlations in 2D-NMR spectra (1H-1H COSY,HSQC anδHMBC) of 1 (Fig. 2),fromwhich the positions of the ester groups were also clarified. The correlations of the protons at δH 5.95 (H-5),4.86 (H-7),and 5.81 (H-14) with the carbonyl carbons at δC169.6,170.7,and165.9 in theHMBCspectrum demonstrated the presences of two acetoxy and one benzoyloxy groups at C-5,C-7,and C-14,respectively. In addition,three slightly weak correlations from methyl signals in acetoxy groups at δH 1.71 (s,3H,2-OAc),2.10 (s,3H,10-OAc),2.15 (s,3H,15-OAc) to three quaternary carbons at δC 87.1 (s,C-2),86.0 (s,C-10),90.2 (s,C-15) (Fig. S5 in Supporting information),respectively,indicated that the three acetoxy groups were located at C-2,C-10 and C-15, respectively.
| Table 1 NMR data for compounds 1 and 2 (TMS as the internal standard,δ in ppm,J in Hz).a,b |
|
Download:
|
| Fig. 2. Key COSY,HMBC,and ROESY correlations of 1. | |
The relative configurations of 1 were decided by the ROESY experiment (Fig. 2) as well as biosynthetic consideration. For the reported natural myrsinol diterpenes,the three rings (5/7/6) forming the myrsinol diterpenoids’ skeleton are trans-fused,H-4 and H2-17 are a-oriented,and Me-16,H-12,the side chain at C-11, and the C-15 acyloxy group are β-oriented [13]. In addition,the ROESY correlations: H-4α with H-3 and H-7 with H-17a,b supported the a-orientations for H-3 and H-7,respectively,and the ROESY correlations between H-12β with H-5,H-14 and Me-20 were in agreement with the β-orientations of H-5,H-14 and Me-20 (Fig. S8 in Supporting information). Consequently,compound 1 was elucidated as 14-desoxo-2α,3β,5α,7β,10,15β-O-hexacetyl- 14α-O-benzoyl-10,18-dihydromyrsinol and given the name euphordracunculin A.
Compound 2,colorless crystals from MeOH,mp 199-203 ℃, [α]D26:5 -119.0 (c 0.18,MeOH),UV (MeOH) λmax (log e): 272 (3.06), 231 (4.12) and 201 (4.10) nm,was found to possess the molecular formula C38H48O14 from the HR-ESI-MS data (m/z 751.2946 [M+Na]+,calcd. 751.2942),indicating 15 degrees of unsaturation. The 1Hand 13CNMRspectra of 2 were similar to those of the known compound 3,except for the absence of an acetoxyl group at C-2. A hydroxyl group at the C-2 position was evident for 2 on the basis of the observation of an upfield shifted quaternary carbon signal (δC 78.4) in the 13C NMR spectrum and HMBC correlations of H-1 (δH 2.45),H-3 (δH 5.12),and Me-16 (δH 1.10) with C-2 (δC 78.4) (Fig. S15 in Supporting information). Further 2D NMR experiments allowed a determination of 2 as 14-desoxo-5α,7b,10,15β-Otetraacetyl- 14α-O-benzoyl-2a-hydroxy-3β-O-propionyl-10,18- dihydromyrsinol and given the name euphordracunculin B. Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21162044) and the Mid-aged and Young Academic and Technical Leader Raising Foundation of Yunnan Province (No. 2010CI040).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.08.005.
| [1] | A.R. Jassbi, Chemistry and biological activity of secondary metabolites in Euphorbia from Iran, Phytochemistry 67 (2006) 1977-1984. |
| [2] | Q.W. Shi, X.H. Su, H. Kiyota, Chemical and pharmacological research of the plants in genus Euphorbia, Chem. Rev. 108 (2008) 4295-4327. |
| [3] | J. Xu, D.Q. Jin, P. Guo, et al., Three new myrsinol diterpenes from Euphorbia prolifera and their neuroprotective activities, Molecules 17 (2012) 9520-9528. |
| [4] | H. Haba, L. Marcourt, M. Benkhaled, C. Long, Minor ent-abietane diterpenoids from Euphorbia guyoniana, Nat. Prod. Commun. 8 (2013) 1519-1522. |
| [5] | S.A. Ivana, P. Milica, M.M. Slobodan, et al., Isolation and biological evaluation of jatrophane diterpenoids from Euphorbia dendroides, J. Nat. Prod. 74 (2011) 1613- 1620. |
| [6] | J.S. Ma, Y.Q. Cheng, Euphorbiaceae (3), Flora of China, Science Press, Beijing, 1997p. 118. |
| [7] | A.M. Zaghloul, New flavonoid glycosides from Euphorbia dracunculoides, Mans. J. Pharm. Sci. 9 (1993) 204-212. |
| [8] | R.K. Gautam, D.K. Mukharaya, Quercetin-3-O-β-D-glucopyranosyl (1→4)-O-α-L-rhamnopyranoside from Euphorbia dracuncoloids Lam. leaves, Nat. Acad. Sci. Lett. (India) 10 (1987) 95-96. |
| [9] | H.M. Chawla, K. Chakrabarty, S.S. Chibber, et al., Chemical components of Euphorbia dracunculoides Lam., Sci. Cult. 48 (1982) 203-205. |
| [10] | H.M. Chawla, K. Chakrabarty, S.S. Chibber, A.N. Kalia, N.C. Chaudhury, Daphnetin from Euphorbia dracunculoides fruits, Indian J. Pharm. Sci. 42 (1980) 138-139. |
| [11] | W.J. Zhang, D.F. Chen, A.J. Hou, Two novel myrinsol diterpenes from Euphorbia prolifera, Chin. Chem. Lett. 13 (2002) 744-747. |
| [12] | J. Li, L. Xu, F.P. Wang, New cytotoxic myrsinane-type diterpenes from Euphorbia prolifera, Helv. Chim. Acta 93 (2010) 746-752. |
| [13] | J. Xu, Y.Q. Guo, C.F. Xie, et al., Bioactive myrsinol diterpenoids from the roots of Euphorbia prolifera, J. Nat. Prod. 74 (2011) 2224-2230. |





