Multicomponent reactions (MCRs) are powerful synthetic tools which have modified the landscape of organic and medicinal chemistry because of environmental concerns by reducing the number of synthetic steps,waste production and energy consumption. MCRs offer the advantage of simplicity insyntheticworkupand efficiency over conventional chemical reactions. This necessitates search and discovery for newer MCRs. Imidazole nucleus has been reported to exhibit variety of biological activities [1, 2, 3].
The incidences of systemic fungal infections are increasing dramaticallydue toanincrease inthenumber ofpatientsundergoing organ transplants,anticancer chemotherapy and patients with AIDS. Commonly used azole antifungal agents are fluconazole, itraconazole,miconazole and voriconazole and have broad-spectrum antifungal activity. These antifungal drugs act by inhibiting CYP51 in the process of biosynthesis of ergosterols through a mechanism in which the heterocyclic nitrogen atom binds to the heme iron atom. However,the increased use of these antifungal drugs has led to the development of resistance to these drugs. Thus, there is an urgent need for development of antifungal agents [4].
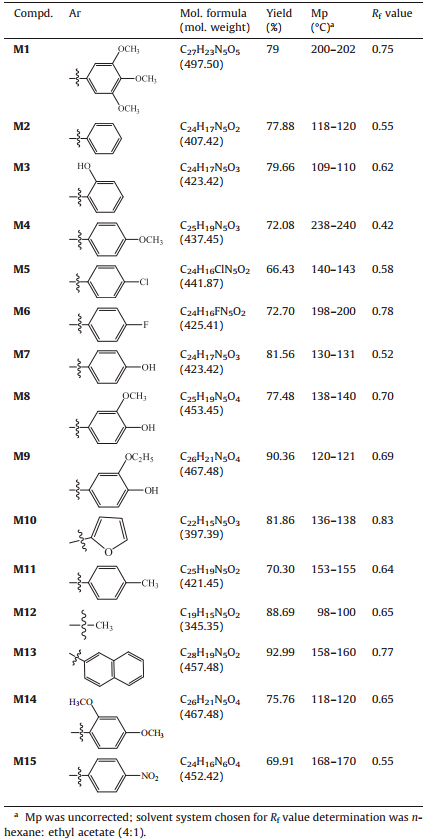
Imidazole drugs have broadened scope in remedying various dispositions in clinical medicines. Imidazole possesses various medicinal properties that include anticancer [5],anticoagulants [6],anti-inflammatory [7, 8],antibacterial and antifungal [9, 10, 11, 12], antiviral [13],anti tubercular [14, 15]. Thus,the high therapeutic properties of the imidazole related drugs have encouraged the medicinal chemists to synthesize a large number of novel chemotherapeutic agents. 1,2,4-Triazole ring has drawn great attention to medicinal chemists due to its wide variety of activities including antibacterial [16, 17, 18] and antifungal [19, 20, 21, 22],anticancer [23, 24] and antioxidant [25, 26]. These two heterocyclic moities are important core for antifungal activity. In the present work,our objective was to design (Fig. 1) and synthesize new compounds having imidazole moiety coupled with 1,2,4-triazole ring with the hope to get enhanced antifungal activity.
|
Download:
|
| Fig. 1. Designing protocol for target compound. | |
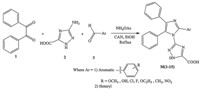
Several methods have been reported for the synthesis of poly substituted imidazoles using variety of catalysts like tandem threecomponent reaction of hydroxylamines,aldehydes and 2-azido acrylates [27]. Various catalysts like BiCl3 [28] and Alumina [29] have been reported for synthesis of tetra substituted imidazoles from benzyl,amines and aldehydes. Ceric (IV) ammonium nitrate (CAN) is a convenient and widely used catalyst for affecting a wide array of synthetic transformations due to its many advantages such as solubility in organic solvents,lowtoxicity,highreactivity andease of handling [30]. Due to our increased interest for search of new antifungal agents having imidazole ring coupled with 1,2,4-triazol, here we report a facile one-pot three component synthesis of novel 3-[(4,5-diphenyl-2-substituted aryl/heteryl)-1H-imidazol-1- yl]-1H-1,2,4-triazole-5-carboxylic acids M(1-15) using ceric ammonium nitrate (CAN) as a catalyst,in good yield as antifungal agent. The activity result and docking study revealed that compounds could be exploited as an antifungal drug.
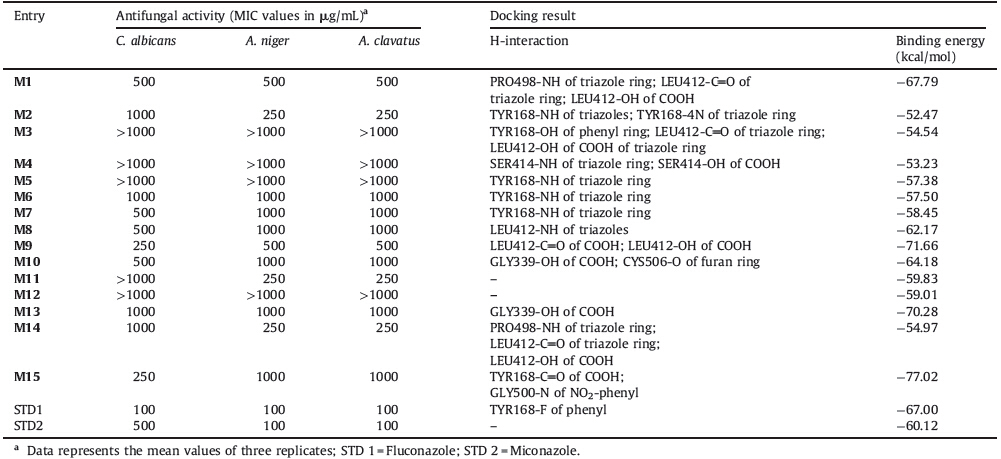
2. Experimental 2.1. ChemistryAll the chemicals used for synthesis were of Merck,Sigma, Research lab,Qualigens and Hi media. Infrared (IR),proton nuclear magnetic resonance (1H NMR) spectra were recorded for the compounds on JASCO FTIR (PS 4000) using KBr pallet,Brucker Avance II (400 MHz) instruments and AVANCE 300 MHz,respectively. Chemical shifts are reported in parts per million (ppm), using TMS as an internal standard. The mass spectra were recorded on 410 Prostar Binary LC with 500 MS IT PDA Detectors. Elemental analyses (C,H,and N) were undertaken with a Shimadzu’s FLASHEA112 analyzer and all analyses were consistent with theoretical values (within ±0.4%),unless indicated. The synthetic protocol employed for the synthesis of 3-[(4,5-diphenyl-2-substituted aryl/heteryl)-1H-imidazol-1-yl]-1H-1,2,4-triazole-5-carboxylic acid is presented in Scheme 1. The purity of the synthesized compounds was checked by TLC and melting points were determined in open capillary tubes and are uncorrected. The physical characterization data of the synthesized compounds are presented in Table 1.
|
Download:
|
| Scheme 1. Synthetic route for a target compounds M(1-15). | |
| Table 1 Physical characterization of the synthesized compounds M (1-15). |
General procedure for the synthesis of 3-[(4,5-diphenyl-2- substituted aryl/heteryl)-1H-imidazol-1-yl]-1H-1,2,4-triazole-5- carboxylic acid M(1-15): A mixture of benzil (0.01 mol),aldehydes (0.01 mol),3-amino-1,2,4-triazole-5-carboxylic acid (0.01 mol), ammonium acetate (0.01 mol) and ceric ammonium nitrate (15 mol%) as a catalyst were refluxed in ethanol (15 mL) for about 3-4 h. The progress of the reaction was monitored by TLC. After completion of reaction,the mixture was cooled to room temperature. The solid formed was filtered and dried. The crude products were recrystallized by ethanol.
2.2. Biological activityThe antifungal activity was evaluated against human pathogenic fungal strains,such as Candida albicans (MTCC 227),Aspergillus clavatus (MTCC 1323),Aspergillus niger (MTCC 282),which are often encountered clinically andwere comparedwith standard drugs like fluconazole and miconazole. From the series of synthesized compounds M(1-15),we have also performed antibacterial activity of 10 selected compounds like M1,M2,M3,M5,M8,M9,M10,M11, M14 and M15. The antibacterial activity was evaluated against strains such as Escherichia coli(MTCC443),Streptococcus pneumoniae (MTCC 109),Staphylococcus aureus (MTCC 96),Streptococcus pyogenes (MTCC 442) and were compared with standard ampicillin. Minimum inhibitory concentration (MIC) values were determined using standard agar method [31].
2.3. Docking studyHomology modeling: The 3D model structure of cytochrome P450 lanosterol 14α-demethylase of C. albicans was built using homology modeling with the help of VLifeMDS 4.3 ProModel as reported by Sangshetti et al. [32]. Amino acid sequence of enzyme was obtained from the Universal Protein Resource (http://www.uniprot.org/) (Accession Code: P10613) and sequence homologous was obtained from Protein Data Bank (PDB) using Blast search. Based on the result of blast search,we used the crystal structure of human lanosterol 14α-demethylase (CYP51) with azole as a template for homology modeling (PDB ID: 3LD6) [33]. The alignment of amino acid sequence of CA-CYP51 (P10613) and human CYP51 (3LD6_B) is given in Fig. S1 (Supporting information). The quality of generated C. albicans lanosterol 14ademethylase model was assessed by using the well-validated program likes PROCHECK [34] and its structural validation is shown in Fig. S2 (Supporting information).
Docking of ligands: The synthesized compounds M(1-15) and standard drug fluconazole and miconazole were docked with the target protein. The 2D structures of synthesized compounds and standard drugs were drawn using VLife2Draw 1.0 and converted to 3D confirmations. The conformers thus obtained, were optimized (MMFF) till they reached a rms gradient energy of 0.001 kcal/(molÅ). The docking of the conformers of each molecule,into the lanosterol 14α-demethylase (CYP51) modeled protein was done by positioning with the active site of cavity 1. The complexes were then minimized using the MMFF method,till they reached an rms gradient of 0.1 kcal/(molÅ). The above procedures were performed using the VLife MDS 4.3 package[35].
3. Results and discussion 3.1. ChemistryAll the titled compounds M(1-15) were synthesized as per the synthetic protocol presented in Scheme 1. Initially,benzil (0.01 mol),benzaldehyde (0.01 mol),3-amino-1,2,4-triazole-5- carboxylic acid (0.01 mol) and ammonium acetate (0.01 mol) were employed as reactants for the model reaction to synthesize compound M2. For the optimization of the reaction conditions,we evaluated the effect of different catalysts for model reaction using ethanol as solvent. A wide variety of catalysts (15 mol%) including oxalic acid,sulphamic acid,boric acid,oxalic acid,ZnO and ceric ammonium nitrate (CAN) were used to test their efficacy for the synthesis of model compound M2. Among the results,best yield was obtained for CAN (92% yield) compared with other catalysts (Table S1,Supporting information).
After deciding the catalyst,the synthetic protocol was extended for synthesis of 3-[(4,5-diphenyl-2-substituted aryl/ heteryl)-1H-imidazol-1-yl]-1H-1,2,4-triazole-5-carboxylic acids M(1-15). Benzil,various aldehydes,3-amino-1,2,4-triazole-5- arboxylic acid and ammonium acetate was refluxed in ethanol using CAN (15 mol%) as a catalyst for about 3-4 h. The purity of the synthesized compounds was checked by TLC and melting points were determined in open capillary tubes melting point apparatus and are uncorrected. The physical data of the synthesized compounds are presented in Table 1. The data obtained from 1H NMR,13C NMR,mass and elemental analysis confirmed the proposed structures (spectral data results are provided in Supporting information). The products were obtained in good yield (80%-93%).
3.2. In vitro antifungal activityThe results of in vitro antifungal activity (Table 2) showed that all the compounds exhibited good to moderate antifungal activity. Amongst the synthesized series some derivatives were found to have moderate activity against C. albicans,A. niger,and A. clavatus as compared with fluconazole. Compounds M9 and M15 showed highest activity against C. albicans when compared with miconazole. The compounds M1,M8 and M10 (MIC = 500 μg/mL against C. albicans) were equipotent when compared with miconazole. The compounds M2,M11 and M14 (MIC = 250 μg/mL against A. niger and A. clavatus) were equipotent when compared with standard drugs.
| Table 2 In vitro antifungal evaluation of the synthesized compounds M(1-15). |
From the antifungal activity data in Table 2,it is observed that scaffolds 1,2,4-triazole and various substituents at aromatic ring were responsible for antifungal activity. Substitution on aromatic aldehyde by 4-OH and 3-OC2H5 as in compound M9 and 4-NO2 group in the compound M15 enhances the antifungal activity.
3.3. In vitro antibacterial activityThe result of in vitro antibacterial activity (Table 3) showed that all the compounds (MIC range = 62.5-500 μg/mL) possess moderate antibacterial activity when compared with ampicillin (MIC range = 100-250 μg/mL). From the series,compound M5 showed higher activity (MIC = 62.5 μg/mL against E. coli),M2 (MIC = 62.5 μg/mL against S. pneumoniae) and M14 (MIC = 62.5 mg/ mL against S. pyogenes)when comparedwith ampicillin. Compound M14 showed higher activity (MIC = 100 μg/mL against S. aureus) when compared with ampicillin. From the antibacterial activity data,phenyl (M2),4-Cl-pheny (M5) and 4-OCH3-phenyl were more active than other derivatives.
| Table 3 In vitro antibacterial evaluation of synthesized compounds M(1-15). |
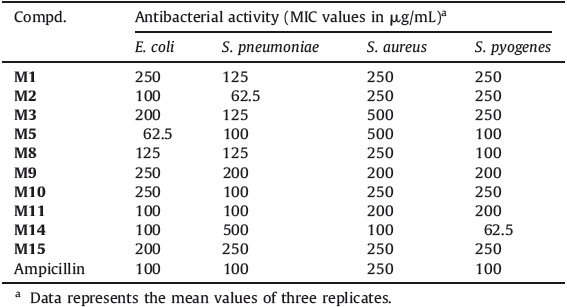
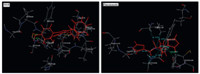
The synthesized compounds M(1-15) and standard drugs (fluconazole and miconazole) were docked into the active site of cytochrome P450 lanosterol 14α-demethylase of C. albicans using VLifeMDS 4.3 software package to understand the binding interactions. The docking calculation and hydrogen bond interactions data obtained are presented in Table 2. The interaction energy of the synthesized compounds and their antifungal activity showed the corresponding results. The active compounds M9 and M15 showed lowest binding interaction energy,i.e. -71.66 and -77.02 kcal/mol,respectively. The docking result indicated that synthesized compounds were held in the active pocket by forming combination of hydrogen bonds,hydrophobic bonds and van der Waals interactions with the enzyme. The docking study also revealed that only triazole ring had formed various hydrogen bonding interactions with enzyme suggesting that triazole ring is more important for inhibiting the 14α-demethylase of C. albicans when in combination with imidazole ring. The -COOH group of triazole ring had formed the various hydrogen bonds with amino acids residue like TYR168,GLY339,LEU412,and SER414. Thus, suggesting that -COOH group is important for antifungal activity. The interactions of compound M15 and fluconazole are shown in Fig. 2. In case of compound M15,amino acids TYR168 and GLY500 had formed hydrogen bonds with O=C of -COOH and nitrogen of -NO2,respectively. On the basis of activity data and docking results,it was found that the compound M15 had potential to inhibit 14α-demethylase of C. albicans.
|
Download:
|
| Fig. 2. Docking of compound M15 and fluconazole. Ligands are shown in red color. Hydrogen bonds are shown in green color. Hydrophobic bonds are shown in sky blue color. | |
The authors are thankful to Mrs. Fatma Rafiq Zakaria,Chairman of Maulana Azad Educational Trust and Dr. Zahid Zaheer,Principal of Y. B. Chavan College of Pharmacy,Dr. Rafiq Zakaria Campus, Aurangabad 431 001 (M.S.),India for providing the laboratory facility.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.10.020.
| [1] | A.A. Marzouk, V.M. Abbasov, A.H. Talybov, Synthesis of 2,4,5-triphenyl imidazole derivatives using diethyl ammonium hydrogen phosphate as green, fast and reusable catalyst, World J. Org. Chem. 1 (2013) 6-10. |
| [2] | P.P. Reddy, K. Mukkanti, K. Purandhar, ALPO4 mediated one-pot, four-component synthesis of 1, 2, 4, 5-tetrasubstituted imidazoles under conventional heating and microwave irradiation, Rasayan J. Chem. 3 (2010) 335-340. |
| [3] | P.J. Das, J. Das, M. Ghosh, Solvent free one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles catalyzed by secondary amine based ionic liquid and defective keggin heteropoly acid, Green Sustain. Chem. 3 (2013) 6-13. |
| [4] | J.H. Block, J.M. Beale (Eds.), Wilson and Griswold's Textbook of Organic Medicinal and Pharmaceutical Chemistry, 11th ed., Lippincott's Williams & Wilkins Publication, 2004, p. 240. |
| [5] | Y. Ozkay, I. Iskdag, Z. Incesu, G. Akalın, Synthesis of 2-substituted-N-[4-(1-methyl- 4,5-diphenyl-1H-imidazole-2-yl)phenyl]acetamide derivatives and evaluation of their anticancer activity, Eur. J. Med. Chem. 45 (2010) 3320-3328. |
| [6] | M.R. Wiley, L.C. Weir, S.L. Briggs, N.Y. Chirgadze, D. Clawson, The design of potent, selective, non-covalent, peptide thrombin inhibitors utilizing imidazole as a S1 binding element, Bioorg. Med. Chem. Lett. 9 (1999) 2767-2772. |
| [7] | A. Puratchikodya, M. Doble, Antinociceptive and antiinflammatory activities and QSAR studies on 2-substituted-4,5-diphenyl-1H-imidazoles, Bioorg. Med. Chem. 15 (2007) 1083-1090. |
| [8] | K.C.S. Achar, K.M. Hosamani, H.R. Seetharamareddy, In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives, Eur. J. Med. Chem. 45 (2010) 2048-2054. |
| [9] | R.V. Shingalapur, K.M. Hosamani, R.S. Keri, Synthesis and evaluation of in vitro anti-microbial and anti-tubercular activity of 2-styryl benzimidazoles, Eur. J. Med. Chem. 44 (2009) 4244-4248. |
| [10] | D. Sharma, B. Narasimhan, P. Kumar, et al., Synthesis, antimicrobial and antiviral evaluation of substituted imidazole derivatives, Eur. J. Med. Chem. 44 (2009) 2347-2353. |
| [11] | D. Zampieri, M.G. Mamolo, L. Vio, et al., Synthesis, antifungal and antimycobacterial activities of new bis-imidazole derivatives, and prediction of their binding to P45014DM by molecular docking and MM/PBSA method, Bioorg. Med. Chem. 15 (2007) 7444-7458. |
| [12] | D. Olender, J. Zwawiak, V. Lukianchuk, et al., Synthesis of some N-substituted nitroimidazole derivatives as potential antioxidant and antifungal agents, Eur. J. Med. Chem. 44 (2009) 645-652. |
| [13] | M. Tonelli, M. Simone, B. Tasso, F. Novelli, V. Boido, Antiviral activity of benzimidazole derivatives. II. Antiviral activity of 2-phenylbenzimidazole derivatives, Bioorg. Med. Chem. 18 (2010) 2937-2953. |
| [14] | P. Gupta, S. Hameed, R. Jain, Ring-substituted imidazoles as a new class of antituberculosis agents, Eur. J. Med. Chem. 39 (2004) 805-814. |
| [15] | J. Pandey, T.K. Vinod, S.S. Verma, et al., Synthesis and antitubercular screening of imidazole derivatives, Eur. J. Med. Chem. 44 (2009) 3350-3355. |
| [16] | G. Nurhan, S. Mevlut, C. Elif, S. Ali, D. Neslihan, Synthesis and antimicrobial activities of some new 1,2,4-triazole derivatives, Turk. J. Chem. 31 (2007) 335-348. |
| [17] | S.F. Barbuceanu, L.A. Gabriela, S. Ioana, D. Constanatin, S. Radu, New S-alkylated 1,2,4-triazoles incorporating diphenyl sulfone moieties with potential antibacterial activity, J. Serb. Chem. Soc. 74 (2009) 1041-1049. |
| [18] | M.R. Banday, A. Rauf, Substituted 1,2,4-triazoles and thiazolidinones from fatty acids spectral characterization and antimicrobial activity, Indian J. Chem. 48 (2009) 97-102. |
| [19] | J.N. Sangshetti, D.B. Shinde, A.P. Sarkate, Synthesis, antifungal activity and docking study of some new 1,2,4-triazole analogs, Chem. Biol. Drug Des. 78 (2011) 800-809. |
| [20] | R. Tang, L. Jin, C. Mou, et al., Synthesis, antifungal and antibacterial activity for novel amide derivatives containing a triazole moiety, Chem. Cent. J. (2013) 7-30. |
| [21] | X. Chai, J. Zhang, Y. Cao, et al., Design, synthesis and molecular docking studies of novel triazole as antifungal agent, Eur. J. Med. Chem. 46 (2011) 3167-3176. |
| [22] | Y. Jiang, J. Zhang, Y. Cao, et al., Synthesis, in vitro evaluation and molecular docking studies of new triazole derivatives as antifungal agents, Bioorg. Med. Chem. Lett. 21 (2011) 4471-4475. |
| [23] | K.S. Bhat, Synthesis and antitumor activity studies of some new fused 1,2,4- triazole derivatives carrying 2,4-dichloro-5-fluorophenyl moiety, Eur. J. Med. Chem. 44 (2009) 5066-5070. |
| [24] | Y.A. Al-Soud, M.N. Al-Dweri, N.A. Al-Masoudi, Synthesis, antitumor and antiviral properties of some 1,2,4-triazole derivatives, Farmaco 59 (2004) 775-783. |
| [25] | P. Valentina, K. Ilango, M. Deepthi, et al., Antioxidant activity of some substituted 1, 2, 4-triazo-5-thione Schiff base, J. Pharm. Sci. Res. 2 (2009) 74-77. |
| [26] | H. Yuksek, S. Kalayli, M.M.O. Mucuk, Synthesis and antioxidant activities of some 4-benzylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives, Ind. J. Chem. 45 (2006) 715-718. |
| [27] | (a) A. Ning, Z. Wang, X. Xu, X. Li, One-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles by a tandem three-component reaction of hydroxyl amines, aldehydes and 2-azido acrylates, ARKIVOC VI (2012) 222-228;(b) J.N. Sangshetti, N.D. Kokare, S.D. Kotharkar, D.B. Shinde, ZrOCl2·8H2O catalyzed one-pot synthesis of 2,4,5-triaryl-1H-imidazoles and substituted 1,4- di(4,5-diphenylimidazol-yl)benzene, Chin. Chem. Lett. 19 (2008) 762-768. |
| [28] | R.K. Sharma, An efficient and one pot synthesis of poly substituted imidazoles catalyzed by BiCl3, Indian J. Chem. 51B (2012) 1489-1493. |
| [29] | A. Saberi, Synthesis of novel highly potent antibacterial and antifungal agents, Asian J. Med. Pharm. Res. 1 (2012) 01-05. |
| [30] | (a) J.N. Sangshetti, N.D. Kokare, S.A. Kotharkar, D.B. Shinde, Ceric ammonium nitrate catalysed three component one-pot efficient synthesis of 2,4,5-triaryl-1Himidazoles, J. Chem. Sci. 120 (2008) 463-467;(b) K.F. Shelke, S.B. Sapkal, M.S. Shingare, Ultrasound-assisted one-pot synthesis of 2,4,5-triarylimidazole derivatives catalyzed by ceric (IV) ammonium nitrate in aqueous media, Chin. Chem. Lett. 20 (2009) 283-287. |
| [31] | D. Greenwood, R.C.B. Slack, J.F. Peutherer, Medical Microbiology, 14th ed., ELBS, London, 1992. |
| [32] | J.N. Sangshetti, F.A.K. Khan, R.S. Chouthe, et al., Synthesis, docking and ADMET prediction of novel 5-((5-substituted-1-H-1,2,4-triazol-3-yl)methyl)-4,5,6,7-tetrahydrothieno[ 3,2-c]pyridine as antifungal agents, Chin. Chem. Lett. 25 (2014) 1033-1038. |
| [33] | N. Strushkevich, S.A. Usanov, H.W. Park, Structural basis of human CYP51 inhibition by antifungal azoles, J. Mol. Biol. 397 (2010) 1067-1078. |
| [34] | R.W. Hooft, G. Vriend, C. Sander, E.E. Abola, Errors in protein structures, Nature 381 (1996) 272. |
| [35] | VLife Molecular Design Suite 4.3, VLife Sciences Technologies Pvt. Ltd; www. Vlifesciences.com. |