c Key Laboratory of Catalysis and Functional Organic Molecule, Chongqing 400067, China;
c Department of Chemistry, Sichuan University, Chengdu 610064, China
Study of new cytochrome P450 monooxygenase model is of great importance to realize marked rate enhancement,high substrate specificity,and distinct reaction selectivity under mild oxidation conditions. In general,the active site of natural monooxygenases is regarded as a hydrophobic cavity or cleft created by folding of the polypeptide chain. To mimic the active site,macrocyclic compounds such as cyclodextrins [1],crown ethers [2, 3],cyclophanes [4] and calixarenes [5],each having an inclusion cavity as a basic skeleton of potent artificial monooxygenase, were usually employed. Our previous studies revealed that the oxa-crown ether substituted hydroxamic cobalt complexes as monooxygenase model showed much more enhanced O2-binding and catalytic oxidation activity than the uncrowned analogs [6, 7]. This inspired us to expand the models to the N-pivot lariat ether type hydroxamic cobalt complexes due to their structural flexibility. However,to the best of our knowledge,no aza crowned hydroxamic metal complexes have been reported yet. A possible reason may be the synthetic difficulty of the key mediate,aza crowned acyl chloride. Giacomelli [8] reported a novel one-flash method without the use of acyl chloride to synthesize hydroxamic acid ligands,which was used herein to the preparation of the dihydroxamic acids functionalized N-pivot lariat ether ligands L1H2-L4H2.
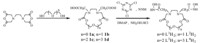
2. ExperimentalThe synthetic route is shown in Scheme 1. The crowned dicarboxylic acids 1a-1d were prepared by our previously disclosed procedure [9]. 1a-1d were treated with cyanuric chloride (TCT) (0.6 equiv.),N-methylmorpholine (NMM) (2 equiv.),and dimethylamino pyridine (DMAP) as a catalyst (0.2 equiv.) in CH2Cl2,followed by hydroxylamine hydrochloride (2.1 equiv.) to obtain ligands L1H2-L4H2. The cobalt complexes were conveniently prepared by treating the ligands L1H2-L4H2 with a stoichiometric amount of Co(OAc)2 in ethanol under N2 atmosphere under reflux.
|
Download:
|
| Scheme 1. Synthetic route of the cobalt complexes with aza-crowned dihydroxamic acid. | |
The oxygenation constants and thermodynamic parameters of CoL1-CoL4 (Table 1) were determined by a reported method [10] (diglyme as solvent,0.99 mol L-1 of pyridine as axial ligand (B), 5 × 10-3 mol L-1 of complexes,9.8 × 104 Pa partial pressure of oxygen and specific temperatures).
| Table 1 Oxygenation constants and thermodynamic parameters (ΔH° and ΔS°). |
The oxidation of p-xylene to p-toluic acid (PTA) catalyzed by CoL1-CoL4 were conducted in a normal gas-liquid apparatus. Air was bubbled into the mixture of p-xylene (40 mL) and CoL (1 × 10-3 mol L-1) with a 2.0Λmin-1 flow rate at 110 ℃. The reaction mixture (0.1 mL) was sampled by pipette periodically before the precipitates appeared (in 5 h),and diluted to 15 mL with ethanol. The accumulative concentration of PTA was determined by the standard acid-base titration. The selective oxidation for PTA was determined by HPLC. The catalytic oxidation performances of CoL1-CoL4 were shown in Table 2.
3. Results and discussionThe new compounds were characterized as follows. L1H2: Yield: 87.9%,m.p. 162-163 ℃,1H NMR (CDCl3): δ 10.32 (s, 2H,OH,D2O exchangeable),4.50 (s,4H,COOCH2),3.72 (s,4H, NCH2CON),3.59 (s,4H,COCH2N),3.11 (s,4H,NCH2CH2N); IR (KBr, cm-1): υmax 3226,3109,1732,1628,1425,1122,965; MS (m/z): 348 (M+); Anal. calcd. for C12H20N4O8: C 41.38,H 5.79,N 16.06; found: C 41.49,H 5.72,N 16.25.
L2H2: Yield: 90.2%,m.p. 147-148 ℃,1H NMR (CDCl3): δ 10.30 (s, 2H,OH,D2O exchangeable),4.39 (m,4H,COOCH2),3.95 (s,4H, NCH2CON),3.66-3.78 (s,8H,COCH2N,COOCH2CH2O),3.25 (s,4H, NCH2CH2N); IR (KBr,cm-1): υmax 3226,3105,1730,1628,1427, 1122,962; MS (m/z): 392 (M+); Anal. calcd. for C14H24N4O9: C 42.86,H 6.17,N 14.28; found: C 42.55,H 6.11,N 14.36.
L3H2: Yield: 86.3%,m.p. 129-130 ℃,1H NMR (CDCl3): δ 10.30 (s,2H,OH,D2O exchangeable),4.37 (t,4H,COOCH2),3.92 (s,4H, NCH2CON),3.64-3.77 (s,8H,COCH2N,COOCH2CH2O),3.60 (s,4H, OCH2CH2O),3.25 (s,4H,NCH2CH2N); IR (KBr,cm-1): υmax 3222, 3106,1732,1630,1425,1120,961; MS (m/z): 436 (M+); Anal. calcd. for C16H28N4O10: C 44.03,H 6.47,N 12.84; found: C 44.15,H 6.37,N 12.97.
L4H2: Yield: 85.1%,m.p. 113-114 ℃,1H NMR (CDCl3): δ 10.28 (s,2H,OH,D2O exchangeable),4.35 (t,4H,COOCH2),3.91 (s,4H, NCH2CON),3.65-3.77 (s,8H,COCH2N,COOCH2CH2O),3.61 (s,8H, OCH2CH2O),3.25 (s,4H,NCH2CH2N); IR (KBr,cm-1): υmax 3225, 3105,1730,1630,1422,1122,960; MS (m/z): 480 (M+); Anal. calcd. for C18H32N4O11: C 45.00,H 6.71,N 11.66; found: C 45.21,H 6.77,N 11.80.
CoL1: Yield: 70.6%,m.p. 261 ℃ (dec.),IR (KBr,cm-1): υmax 3112, 1732,1595,1420,1122,982; Anal. calcd. for C12H18N4O8Co: C 35.57,H 4.48,N 13.83,Co 14.54; found: C 35.39,H 4.39,N 14.03,Co 14.70. Λm (S cm2 mol-1): 2.56. CoL2: Yield: m.p. 248 ℃ (dec.),IR (KBr,cm-1): υmax 3109,1733, 1601,1421,1122,978; Anal. calcd. for C14H22N4O9Co: C 37.43,H 4.94,N 12.47,Co 13.12; found: C 37.31,H 5.02,N 12.58,Co 13.56. Λm (S cm2 mol-1): 5.71.
CoL3: Yield: m.p. 227 ℃ (dec.),IR (KBr,cm-1): υmax 3110,1733, 1605,1422,1120,978; Anal. calcd. for C16H26N4O10Co: C 38.95,H 5.31,N 11.36,Co 11.95; found: C 38.77,H 5.46,N 11.19,Co 12.18. Λm (S cm2 mol-1): 7.26.
CoL4: Yield: 64.1%,m.p. 221 ℃ (dec.),IR (KBr,cm-1): υmax 3107, 1731,1607,1420,1122,979; Anal. calcd. for C18H30N4O11Co: C 40.23,H 5.63,N 10.43,Co 10.97; found: C 40.15,H 5.47,N 10.64,Co 11.21. Λm (S cm2 mol-1): 4.85.
The oxygenation constants in Table 1 were of significant difference. The order of the O-binding activity is: CoL3 > CoL2 > CoL4 > CoL1,which should be attributed to the structural diversity. Since the only difference among the cobalt complexes was the size of the crown ether ring (or the number of the ethyleneoxy linkers), it could be deduced that the macrocyclic effect of crown ring, which possesses a special configuration,would favor oxygen molecule to approach the active center of the Co(II) complexes and form the Co-O2 bond through its hydrophobic outer ethylene groups and orderly arranged inner aza oxa atoms. However,CoL4, which possesses the largest crown ring,did not show the highest O2-binding activity. The reason may that the large crown ring of CoL4 would be distorted and create steric hindrance around the active center,which is disadvantageous for the binding of O2 [11]. Moreover,the ΔH° seems to contribute to the formation of O2-Co(II) bond. The Co(II) complexes with smallerΔH° show larger oxygenation constants. The relevance might be used to judge the O2-binding activity of CoL.
As shown in Table 2,The oxidation of p-xylene catalyzed by CoL1-CoL4 experienced a certain induction period (0.3-0.4 h), which indicated there may be a connection between the dioxygen affinity and induction period and the coordination of molecular oxygen to the central cobalt ions is necessary to the initiation of the catalytic oxidation reaction. Meanwhile,same as the order of the O2-binding activity of CoL1-CoL4,CoL3 showed the highest catalytic oxidation activity and selectivity followed by CoL2,and then CoL4 and CoL1. This result indicated that on one hand,a proper size of crown ether ring could offer a favorable microenvironment or even some kind of substrate specificity to significantly enhance the catalytic oxidation performance of the cobalt complexes. On the other hand,the steric hindrance of the crown ether ring would shield the active center,which is not advantageous to the formation of the active oxygen affinity species and the inclusion to the substrate. It also seemed to indicate that the crown ring configuration played an important role in the modulation of the catalytic oxidation,which would be researched in our future structural studies of the cobalt complexes.
| Table 2 The catalytic oxidation performances of CoL. |
In this paper,a novel type of aza crowned hydroxamic acids cobalt(II) complexes as a biomimetic oxygen carrier were synthesized and characterized. The O2-binding and catalytic activity of the complexes indicated that the size and configuration of the aza crown ring play an important role in the modulation of the oxygen-activating performance.
AcknowledgmentThis work was supported by Chongqing Education Commission (No. KJ1400632).
| [1] | R. Brelow, S.D. Dong, Biomimetic reaction catalyzed by cyclodextrins and their derivatives, Chem. Rev. 98 (1998) 1997-2011. |
| [2] | C.J. Pederson, The discovery of crown ether, Angew. Chem. Int. Ed. Engl. 27 (1988) 1021-1027. |
| [3] | X.Y. Li, C. Hu, M.L. Ma, et al., Xylyl derived oxacalixcrowns: synthesis and crystal structure, Chin. Chem. Lett. 24 (2013) 279-282. |
| [4] | C. Seel, F. Vigtle, Molecules with large cavities in supramolecular chemistry, Angew. Chem. Int. Ed. Engl. 31 (1992) 528-549. |
| [5] | J.H. Fendler, Membrane Mimetic Chemistry, John Wiley & Sons, New York, 1982. |
| [6] | X.Y. Wei, S.Y. Qin, Dioxygen affinities and catalytic oxidation performance of Co(Ⅱ) complexes with phenol ether bridged dihydroxamic acids, Chin. Chem. Lett. 17 (2006) 1259-1262. |
| [7] | X.Y. Wei, S.Y. Qin, Dioxygen affinities and catalytic oxidation performance of cobalt(Ⅱ) dihydroxamic acids with central function group, React. Kinet. Catal. Lett. 95 (2008) 337-344. |
| [8] | G. Giacomelli, A. Porcheddu, M. Salaris, Simple one-flask method for the preparation of hydroxamic acid, Org. Lett. 5 (2003) 2715-2717. |
| [9] | A.J. Zhang, S.Y. Qin, Improvement in synthesis of N,N'-dicarboxymethyl macrocyclic ether-bislactones, Chem. Online 9 (2000) 34-35. |
| [10] | D. Chen, A.E. Martell, Dioxygen affinities of synthetic cobalt Schiff base complexes, Inorg. Chem. 26 (1987) 1026-1030. |
| [11] | H. Yang, S.Y. Qin, X.X. Lu, Dioxygen affinities and catalytic epoxidation performance of transition-metal hydroxamates, Chin. Chem. Lett. 10 (1999) 845-848. |