As strong agonists of the purinergic receptors, endogenous dinucleoside pentaphosphates, including Ap5A, Ap5G, and Gp5G, are essential extracellular signaling molecules, and play important roles in physiological and pathological processes in cardiovascular and nervous systems [1]. Ap5A [2] and Ap5G [2b,3] exhibit both vasoregulatory effect and proliferative effect on vascular smooth muscle cells (VSMCs), and Gp5G [3,4] only induces proliferation of VSMCs. In biochemical research, the Ap5A-Zn2+ complex has been utilized as a probe to elucidate the configurational requirement for adenylate kinase catalysis [5]. In addition, artificial dinucleoside pentaphosphates, such as Up5U and Ap5T, have also been synthesized. While Up5U showed selective agonist activity on P2Y2 receptor [6], Ap5T exhibited strong inhibitory effects on thymidine kinase, thymidylate kinase, and ribonucleotide reductase [7].
Currently, the available synthetic methods for the preparation of dinucleoside pentaphosphates are still limited compared to those for dinucleoside di-, tri-, and tetraphosphates. The most commonly employed strategy was the direct condensation of nucleoside diphosphate with another molecule of nucleoside triphosphate by using N,N0-dicyclohexylcarbodiimide (DCC) or N,N0-carbonyldiimidazole (CDI) [5a,6-8]. But this method was typically low yielding (10%-20%) and time-consuming (1-3 days). Though Jones et al. [9] and Taylor et al. [10] reported novel methods for dinucleoside pentaphosphate synthesis, the protection of the nucleosides and preparation of specific condensing reagents limited their values in practical applications. On the basis of the P(V)-N activation strategy we developed for the synthesis for nucleoside polyphosphates [11], we report in this paper a general and facile approach for the synthesis of dinucleoside pentaphosphates (Np5N's) via the 4,5-dicyanoimidazole (DCI)-promoted coupling of nucleoside 5'-phosphoropiperidates with nucleoside 5'-tetraphosphates (Np4s). The P(V)-N activation method for the efficient synthesis of Np4s [12] is also described.
2. ExperimentalAll reactions were performed in anhydrous solvents under an atmosphere of dry argon. The triethylammonium salts of nucleoside 5'-phosphoropiperidates were synthesized according to the procedure described in a previous report [11b]. Tris(tetra-nbutylammonium) dihydrogen triphosphate was prepared according to a known method [12a]. Ion exchange chromatography employed DEAE Sephadex A-25 exchanger. Preparative HPLC was equipped with a RP C18 column (19 mm × 250 mm, 10 μm). NMR spectra were obtained with a 400 MHz instrument with chemical shifts reported in parts per million (ppm, δ). IR spectra were recorded on a FT-IR spectrometer. Low-resolution mass spectra were obtained with an ion trap mass spectrometer and reported as m/z.
2.1. General procedure for the synthesis of nucleoside5'-tetraphosphates (5-8) To a solution of nucleoside 5'-phosphoropiperidate (0.1 mmol) in DMF (2 mL) were added tris(tri-n-butylammonium) dihydrogen triphosphate (0.2 mmol) and 4,5-dicyanoimidazole (DCI, 0.6 mmol). The reaction was stirred at 20 ℃ for 12 h and concentrated in vacuo. The residue was dissolved in NaOAc aqueous solution (3 mol/L, 1 mL) and EtOH (50 mL) was added. The resulting white precipitate was collected by centrifuge. The crude product was dissolved in deionized H2O (1 mL) and loaded on a DEAE Sephadex A-25 ion exchange column (1.6 cm × 25 cm). Elution with NH4HCO3 buffer (linear gradient 0.3-0.6 mol/L), combination of appropriate fractions, and lyophilization afforded Np4 in ammonium salt form. For characterization, passage of the solution of the ammonium salt in deionized H2O through a bed of Dowex 50W-X8 ion exchange resin (Na+ form) and lyophilization afforded nucleoside 5'-tetraphosphate as pentasodium salt. For the next step reaction, to the ammonium salt in deionized H2O was added tetra-n-butylammonium hydroxide (3 equiv.), and the solution was repeatedly evaporated with deionized H2O (1 mL × 3) to afford nucleoside 5'-tetraphosphate as more soluble tris(tetra-n-butylammonium) salt.
Uridine 5'-tetraphosphate, pentasodium salt (5): Starting from 1 (49 mg), 5 (45 mg, 67%) was obtained as a white solid. 1H NMR (400 MHz, D2O): δ 7.99 (d, 1H, J = 8.0 Hz), 6.06-5.98 (m, 2H), 4.5'- 4.40 (m, 2H), 4.35-4.30 (m, 1H), 4.30-4.23 (m, 2H); 13C NMR (100 MHz, D2O): δ 166.2, 152.0, 141.7, 102.8, 88.1, 83.6 (d, JP,C = 9.0 Hz), 73.7, 69.8, 65.2 (d, JP,C = 5.1 Hz); 31P NMR (D2O, 162 MHz): δ -8.0 (d, 1P, J = 18 Hz), -11.1 (dd, 1P, J = 18 Hz), -22.3 (dd, 1P, J1 = J2 = 18 Hz), -22.5 (d, 1P, J1 = J2 = 18 Hz); IR (cm-1): υmax 3336, 2987, 2899, 1695, 1413, 1228, 1087, 924, 838; LRMS (ESI-): m/z calcd. for C9H15N2O18P4 [M-H]- 562.9; found: 563.0.
Cytidine 5'-tetraphosphate, pentasodium salt (6): Starting from 2 (49 mg), 6 (41 mg, 61%) was obtained as a white solid. 1H NMR (400 MHz, D2O): δ 7.96 (d, 1H, J = 7.2 Hz), 6.14 (d, 1H, J = 7.4 Hz), 6.01 (d, 1H, J = 4.4 Hz), 4.43-4.37 (m, 1H), 4.36-4.30 (m, 1H), 4.29- 4.20 (m, 3H); 13C NMR (100 MHz, D2O): δ 166.3, 157.9, 141.7, 96.9, 89.0, 83.1 (d, JP,C = 8.7 Hz), 74.3, 69.6, 65.0 (d, JP,C = 5.6 Hz); 31P NMR (D2O, 162 MHz): δ -9.4 (d, 1P, J = 18 Hz), -14.4 (d, 1P, J = 18 Hz), -24.9 (dd, 1P, J1 = J2 = 18 Hz), -25.7 (dd, 1P, J1 = J2 = 18 Hz); IR (cm-1): υmax 3370, 2985, 2894, 1693, 1537, 1418, 1235, 1079, 887; LRMS (ESI-): m/z calcd. for C9H16N3O17P4 [M-H]- 561.9; found: 562.0.
Adenosine 5'-tetraphosphate, pentasodium salt (7): Starting from 3 (52 mg), 7 (45 mg, 65%) was obtained as a white solid. 1H NMR (400 MHz, D2O): δ 8.54 (s, 1H), 8.24 (d, 1H, J = 6.5 Hz), 6.14 (d, 1H, J = 6.1 Hz), 4.72-4.70 (m, 1H), 4.64 (dd, 1H, J1 = J2 = 4.8 Hz), 4.48-4.40 (m, 1H), 4.36-4.27 (m, 1H), 4.27-4.18 (m, 1H); 13C NMR (100 MHz, D2O): δ 155.6, 152.8, 149.2, 139.9, 118.6, 86.5, 84.3 (d, JP,C = 9.0 Hz), 74.2, 70.4, 65.4 (d, JP,C = 5.3 Hz); 31P NMR (D2O, 162 MHz): δ -11.5 (d, 1P, J = 18 Hz), -14.8 (d, 1P, J = 18 Hz), -25.8 (dd, 1P, J1 = J2 = 18 Hz), -26.2 (dd, 1P, J1 = J2 = 18 Hz); IR (cm-1): υmax 3354, 3049, 2795, 1714, 1492, 1453, 1260, 1087, 1044, 967, 924; LRMS (ESI-): m/z calcd. for C10H16N5O16P4 [M-H]- 586.0; found: 586.1. Guanosine 5'-tetraphosphate, pentasodium salt (8): Starting from 4 (54 mg), 8 (46 mg, 64%) was obtained as a white solid. 1H NMR (400 MHz, D2O): δ 8.14 (s, 1H), 5.93 (d, 1H, J = 6.0 Hz), 4.75- 4.70 (m, 1H), 4.65-4.58 (m, 1H), 4.43-4.33 (m, 1H), 4.31-4.16 (m, 2H); 13C NMR (100 MHz, D2O): δ 159.2, 154.1, 152.0, 138.0, 116.4, 86.7, 84.4, 73.7, 70.6, 65.6; 31PNMR(D2O, 162 MHz): δ-10.4 (d, 1P, J = 19 Hz), -14.8 (d, 1P, J = 19 Hz), -25.6 (dd, 1P, J1 = J2 = 18 Hz), -26.2 (d, 1P, J1 = J2 = 18 Hz); IR (cm-1): υmax 3378, 2960, 2950, 1712, 1673, 1543, 1403, 1255, 1065, 914, 812; LRMS (ESI-): m/z calcd. for C10H16N5O17P4 [M-H]- 601.9; found: 602.0.
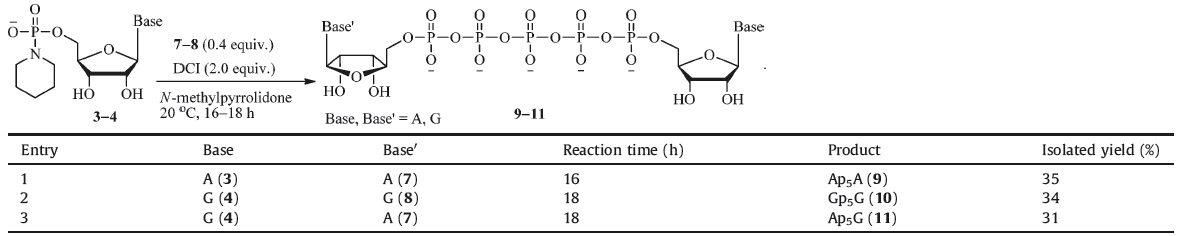
2.2. General procedure for the synthesis of dinucleoside-50,5'-pentaphosphates (9-11)To a solution of nucleoside 5'-phosphoropiperidate (0.1 mmol) in N-methylpyrrolidone (2 mL) were added nucleoside 5'-tetraphosphate (tetra-n-butylammonium salt, 0.04 mmol) and DCI (0.2 mmol). The reaction was stirred at 20 ℃ for 16-18 h. The white precipitation was collected by centrifuge. The crude product was dissolved in deionized H2O (0.5 mL) and loaded on a DEAE Sephadex A-25 ion exchange column (1.6 cm × 25 cm). Elution with NH4HCO3 buffer (linear gradient 0.5 to 0.9 mol/L), combination of appropriate fractions, and lyophilization afforded dinucleoside pentaphosphate in ammonium salt form. To remove the small amount of contaminated polyphosphate byproducts, the ammonium salt was further purified by a preparative RP HPLC [flow rate = 20 mL/min; linear gradient of 0-10% MeOH in TEAB buffer (10 mmol/L, pH 8.0) over 15 min; UV detection at 254 nm]. Combination of appropriate fractions and lyophilization afforded dinucleoside pentaphosphate in triethylammonium salt form. Passage of the solution of the triethylammonium salt in deionized H2O through a bed of Dowex 50W-X8 ion exchange resin (Na+ form) and lyophilization afforded dinucleoside pentaphosphate as pentasodium salt.
P1,P5-Diadenosine-50,5'-pentaphosphate, pentasodium salt (9): Starting from 7 (52 mg), 9 (14 mg, 35%) was obtained as a white solid. 1H NMR (400 MHz, D2O): δ 8.38 (s, 2H), 8.11 (d, 2H, J = 6.0 Hz), 6.06-5.98 (m, 2H), 4.75-4.68 (m, 2H), 4.60-4.51 (m, 2H), 4.39-4.32 (m, 2H), 4.31-4.15 (m, 4H); 13C NMR (100 MHz, D2O): δ 154.4, 151.8, 148.1, 138.9, 117.3, 85.7, 73.7, 69.4, 67.6, 64.3; 31P NMR (D2O, 162 MHz): δ -11.5 (m, 2P), -22.9 (m, 3P); IR (cm-1): υmax 3324, 3149, 2975, 1714, 1492, 1404, 1260, 1087, 1044, 967, 924; LRMS (ESI-): m/z calcd. for C20H28N10O55P5 [M-H]- 915.0; found: 915.1.
P1,P5-Diguanosine-50,5'-pentaphosphate, pentasodium salt (10): Starting from 8 (52 mg), 10 (14 mg, 34%) was obtained as a white solid. 1H NMR (400 MHz, D2O): δ 8.00 (s, 2H), 5.80 (s, 2H), 4.60-4.44 (m, 4H), 4.35-4.15 (m, 6H); 13C NMR (100 MHz, D2O): δ 158.6, 153.5, 151.3, 137.4, 115.8, 86.4, 83.6, 73.1, 70.0, 65.0; 31P NMR (D2O, 162 MHz): δ -11.0 (m, 2P), -22.4 (m, 3P); IR (cm-1): υmax 3388, 2964, 2922, 1673, 1543, 1413, 1250, 1065, 914, 816; LRMS (ESI-): m/z calcd. for C20H28N10O24P5 [M-H]- 947.0; found: 947.1.
P1-Adenosine-5'-P5-guanosine-5'-pentaphosphate, pentasodium salt (11): Starting from 7 (52mg), 11 (13 mg, 31%) was obtained as a white solid. 1H NMR (400 MHz, D2O): δ 8.33 (s, 1H), 8.04 (s, 1H), 7.91 (s, 1H), 5.95 (d, 1H, J = 5.8 Hz), 5.70 (d, 1H, J = 6.3 Hz), 4.63 (m, 2H), 4.45 (m, 2H), 4.25-4.20 (m, 2H), 4.13-4.10 (m, 4H); 13C NMR (100 MHz, D2O): δ 159.0, 155.6, 154.0, 153.0, 151.8, 149.2, 140.1, 137.9, 118.6, 116.3, 86.8 (×2), 84.2 (×2), 74.4, 73.5, 70.5 (×2), 65.5 (×2); 31PNMR (162 MHz,D2O): δ-11.0(m, 2P), -22.8 (m, 3P); IR (cm-1): υmax 3783, 3455, 2950, 2673, 2480, 1722, 1706, 1618, 1442, 1380, 1243, 1122, 1060, 948, 800, 732; LRMS (ESI-): m/z calcd. for C20H28N10O23P5 [M-H]- 931.0; found: 931.1.
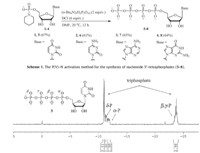
3. Results and discussionAs shown in Scheme 1, nucleoside 5'-tetraphosphates (5-8) were efficiently synthesized by treating nucleoside 5'-phosphoropiperidates (1-4) with 2.0 equiv. of tris(tetra-n-butylammonium) triphosphate and 6.0 equiv. of DCI in anhydrous DMF at 20 ℃ for 12 h. The precipitated crude products were separated by filtration. Ethanol precipitation of the sodium salts followed by ion exchange chromatography afforded 5-8 in good isolated yields ranging from 61% to 67%. The 31P NMR spectrum of crude reaction mixture of 5 (Fig. 1) showed that the conversion of 1-5 was smooth and clean. The calculated 31P NMR yield of 5 was close to 90%. Compared to nucleoside 5'-triphosphate synthesis [11a], longer reactiontimewas required, indicating that the nucleophilicity of triphosphate reagent was lower than that of pyrophosphate. However, due to the highly negatively charged nature, the isolated yields of 5-8 were slightly lower than those of nucleoside di- and tri-phosphates. It was also noticed that Np4s were less stable than their NTP counterparts. When the aqueous solution of 7 was stored at -20 ℃, about 10% of 7 decomposed after 6 months.
|
Download:
|
| Fig. 1. The 31P NMR spectrum of crude reaction mixture of Up4 (5). | |
In the following research, adenosine 5'-phosphoropiperidate (3) was coupled with Ap4 (7) in 1:0.5 molar ratio [11d] in Nmethylpyrrolidone at 20 ℃ with or without DCI. 31P NMR tracing experiments showed that there was almost no reaction between the reactants after 24 h without DCI. This result was in accordance with the observed low reactivity of phosphoropiperidate toward phosphate nucleophiles in our previous research. In contrast, addition of acidic DCI (2.0 equiv.) promoted the reaction to completion in 16 h. But when 3 disappeared, there was still significant amount of 7 (~20%) unreacted due to its low nucleophilicity and the self-condensation of 3. Decrement of the equivalent of 7 to 0.4 effectively promoted its consumption, and the 31P NMR yield of Ap5A (9) was improved to around 50%. But we found that ion exchange chromatography alone was difficult to completely separate the desired Np5N0 products from other closely related long chain polyphosphate impurities. Therefore, RP-HPLC was employed afterward to ensure the purity of these highly negatively charged compounds. As shown in Table 1, three naturally occurring Np5N's (9-11) were synthesized and isolated in 31%-35% yields following this P(V)-N activation method.
| Table 1 The P(V)–N activation method for the synthesis of dinucleoside pentaphosphates (9–11). |
In summary, we developed efficient P(V)-N activation methods for the synthesis of both nucleoside 5'-tetraphosphates and dinucleoside pentaphosphates. Compared to the known methods, these new approaches feature easily accessible starting materials, mild reaction conditions, and moderate to good isolated yields, and provide facile and reliable access to Np4s and Np5N's.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 21002041 and 21262014), Key Project of Chinese Ministry of Education (No. 212092), Scientific Research Foundation of Chinese Ministry of Human Resources and Social Security for Returned Chinese Scholars (2011), and Research Funds (Nos. ky2012zy08 and 2013QNBJRC001) and Startup Funds for PhDs (2010) from JXSTNU for financial support.
| [1] | (a) V. Jankowski, M. van der Giet, H. Mischak, et al., Dinucleoside polyphosphates: strong endogenous agonists of the purinergic system, Br. J. Pharmacol. 157 (2009) 1142-1153;(b) E.G. Delicado, M.T. Miras-Portugal, L.M. Carrasquero, et al., Dinucleoside polyphosphates and their interaction with other nucleotide signaling pathways, Pflugers Arch. 452 (2006) 563-572. |
| [2] | (a) H. Schlüter, E. Offers, G. Brüggemann, et al., Diadenosine phosphates and the physiological control of blood pressure, Nature 367 (1994) 186-188;(b) M. van der Giet, S. Schmidt, M. Tölle, et al., Effects of dinucleoside polyphosphates on regulation of coronary vascular tone, Eur. J. Pharmacol. 448 (2002) 207-213;(c) V. Jankowski, S. Karadogan, R. Vanholder, et al., Paracrine stimulation of vascular smooth muscle proliferation by diadenosine polyphosphates released from proximal tubule epithelial cells, Kidney Int. 71 (2007) 994-1000. |
| [3] | M. van der Giet, T. Westhoff, O. Cinkilic, et al., The critical role of adenosine and guanosine in the affinity of dinucleoside polyphosphates to P(2X)-receptors in the isolated perfused rat kidney, Br. J. Pharmacol. 132 (2001) 467-474. |
| [4] | J. Jankowski, V. Jankowski, B. Seibt, et al., Identification of dinucleoside polyphosphates in adrenal glands, Biochem. Biophys. Res. Commun. 304 (2003) 365-370. |
| [5] | (a) N. Stern, D.T. Major, H.E. Gottlieb, et al., What is the conformation of physiologically active dinucleoside polyphosphates in solution? Conformational analysis of free dinucleoside polyphosphates by NMR and molecular dynamics simulations, Org. Biomol. Chem. 8 (2010) 4637-4652;(b) K.A. Henzler-Wildman, V. Thai, M. Lei, et al., Intrinsic motions along an enzymatic reaction trajectory, Nature 450 (2007) 838-844. |
| [6] | W. Pendergast, B.R. Yerxa, J.G. Douglass, et al., Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5'-polyphosphates, Bioorg. Med. Chem. Lett. 11 (2001) 157-160. |
| [7] | L.C. Davies, J.A. Stock, S.E. Barrie, et al., Dinucleotide analogues as inhibitors of thymidine kinase, thymidylate kinase, and ribonucleotide reductase, J. Med. Chem. 31 (1988) 1305-1308. |
| [8] | (a) P. Feldhau, T. Fröhlich, R.S. Goody, et al., Synthetic inhibitors of adenylate kinases in the assays for ATPases and phosphokinases, Eur. J. Biochem. 57 (1975) 197-204;(b) J. Köhrle, K.S. Boos, E. Schlimme, Preparation of [14C]-P1,P5-di(adenosine 50- )pentaphosphate by direct reaction of [14C]-adenosine 50-diphosphate with activated adenosine 50-triphosphate, Liebigs Ann. Chem. (1977) 1160-1166;(c) A. Hampton, F. Kappler, D. Picker, Species- or isozyme-specific enzyme inhibitors. 4. Design of a two-site inhibitor of adenylate kinase with isozyme selectivity, J. Med. Chem. 25 (1982) 638-644. |
| [9] | Q. Han, B.L. Gaffney, R.A. Jones, One-flask synthesis of dinucleoside tetra- and pentaphosphates, Org. Lett. 8 (2006) 2075-2077. |
| [10] | S. Mohamady, S.D. Taylor, Synthesis of nucleoside tetraphosphates and dinucleoside pentaphosphates via activation of cyclic trimetaphosphate, Org. Lett. 15 (2013) 2612-2615. |
| [11] | (a) Q. Sun, S.S. Gong, J. Sun, et al., A P(V)-N activation strategy for synthesis of nucleoside polyphosphates, J. Org. Chem. 78 (2013) 8417-8426;(b) Q. Sun, S.S. Gong, J. Sun, et al., Efficient synthesis of nucleoside 5'-triphosphates and their β,γ-bridging oxygen-modified analogs from nucleoside 5'-phosphates, Tetrahedron Lett. 55 (2014) 2114-2118;(c) Q. Sun, J. Sun, S.S. Gong, et al., Efficient synthesis of 5-hydroxymethyl-, 5- formyl-, and 5-carboxyl-2'-deoxycytidine and their triphosphates, RSC Adv. 4 (2014) 36036-36039;(d) Q. Sun, S.S. Gong, S. Liu, et al., 4,5-Dicyanoimidazole-promoted synthesis of dinucleoside polyphosphates and their analogs, Tetrahedron 70 (2014) 4500-4506;(e) Q. Sun, X.J. Li, J. Sun, et al., An improved P(V)-N activation strategy for the synthesis of nucleoside diphosphate 6-deoxy-L-sugars, Tetrahedron 70 (2014) 294-300;(f) Q. Sun, S. Liu, J. Sun, et al., An H-phosphonate strategy for the synthesis of 2', 3'-dideoxynucleoside triphosphates and homodinucleotides, Chin. Chem. Lett. 25 (2014) 427-430. |
| [12] | (a) A.R. Kore, Z. Xiao, A. Senthilvelan, et al., An efficient synthesis of pyrimidine specific 2'-deoxynucleoside-5'-tetraphosphates, Nucleosides Nucleotides Nucleic Acids 31 (2012) 567-573;(b) A.R. Kore, A. Senthilvelan, M. Shanmugasundaram, A new, facile, and protection- free one-pot chemical synthesis of 2'-deoxynucleoside-5'-tetraphosphates, Tetrahedron Lett. 53 (2012) 5868-5870. |