Oxidation reaction is one of the most important transformations in organic synthesis [1]. However,these reactions essentially involve the use of stoichiometric oxidants,leading to large amounts of waste that are not at all environmentally benign. An oxidation process with molecular oxygen or in air,is called aerobic oxygenation which addresses these issues in both economic and environmental ways [2]. Since air or pure oxygen is inexpensive while water is the only byproduct,aerobic oxygenations are comparatively greener and more preferable.
Benzothiazoles are common building blocks in many pharmaceuticals that exhibit remarkable biological and therapeutic activities. For example,benzothiazole derivatives have been used for diabetes [3],antitumor drugs [4] and amyloid inhibitors for treatment of Alzheimer’s disease [5]. As a consequence,much effort has been devoted to the preparation of benzothiazoles. Several synthetic methodologies have been developed over the years. In the most cases,catalytic amounts of cyanide [6],metal oxide nanocrystals [7],chlorobenzene [8],copper (I) [9],and an excess of oxidants,such as POCl3 [10],K2S2O8 [11] and H2O2 [12, 13] are required. They also suffer from some bottlenecks such as the use of toxic and expensive reagents or catalysts,long reaction time,harsh reaction conditions,side reactions and low yields. Far less is known,however,about aerobic oxidative cyclizations forming benzothiazoles. The development of an efficient and facile synthetic route to benzothiazoles in air is becoming increasingly timely.
Herein,we report the synthesis of benzothiazole derivatives via a reaction of o-aminothiophenol with various aldehydes catalyzed by yttrium chloride in air (Scheme 1). This methodology successfully combines C-H bond cleavage,dioxygen activation and oxidative C-N bond functionalization in terms of green chemistry and atom economy. We demonstrated that yttrium chloride plays a decisive role in aerobic oxidation.

|
Download:
|
| Scheme 1. The process to benzothiazole derivatives. | |
1H NMR and 13C NMR spectrums were made on Bruker ARX 400 for proton. Melting points were recorded on a WRS-2 data microscopic melting point apparatus and were uncorrected. Flash column chromatography was carried out using silica gel (200- 300). All reagents were obtained from commercial sources and used as received.
A general procedure for preparation of dihydropyrazines 3: YCl3 (0.0098 g,0.05 mmol) were dissolved in 10 mL EtOH and stirred until the solid dissolved completely in refluxing,then 2- aminobenzenethiols 1a (0.118 mL,1.1 mmol) and benzaldehyde 2a (0.101 mL,1.0 mmol) was added into the reaction mixture. After 30 min,the reaction was complete monitored by TLC analysis. The reaction mixture was cooled to room temperature,and then evaporated in vacuum. The product purified by column chromatography (PE-EtOAc = 20:1) to give white solid 3a (0.2092 g,99%). Other products were synthesized through the same procedure. All 1H NMR and 13C NMR results were summarized in Supporting information. The configuration of compounds 3a-3o was assigned by comparing 1H NMR and 13C NMR data with known compounds [14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26]. 3. Results and discussion
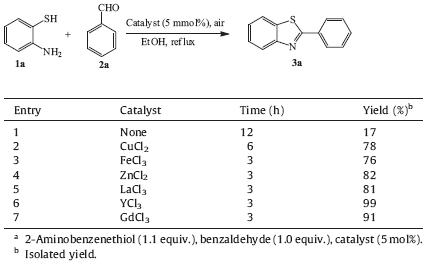
Initially,2-aminobenzenethiols (1a) and benzaldehyde (2a) were chosen as the model reactants to detect the reaction conditions. The screening of different parameters was summarized in Table 1. It turned out that in the absence of the catalysts, the product was obtained in only 17% yield (Table 1,entry 1). In the presence of traditional catalysts such as CuCl2,FeCl3 or ZnCl2, the yields were dramatically improved (Table 1,entries 2-4). Interesting,the reaction appears to work more efficiently by using rare earth chlorides (Table 1,entries 5-7). Among the five selected catalysts,YCl3 has been proved to be the most effective (Table 1,entry 6). Alcohol is the best solvent for catalyzed synthesis of 3a in air.
| Table 1 Catalyst-screen for the synthesis of 3aa |
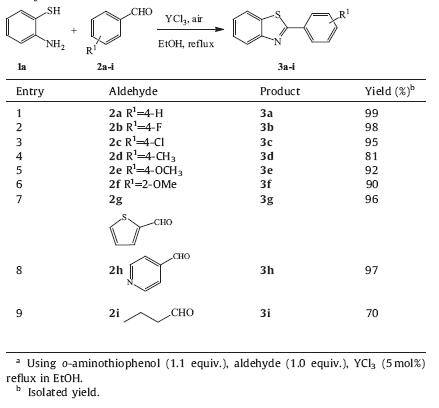
Encouraged by the above experiments,we further explored the synthetic protocol and the scope by employing different kinds of aldehydes functionalized with electron-rich and electron-deficient groups. The results were summarized in Table 2. Clearly,all reactions worked well irrespective of the substituents on the aldehydes substrates (Table 2,entries 1-8). It is noteworthy that the reaction with aliphatic aldehyde (Table 2,entry 9) also afforded the desired product in satisfactory yield.
| Table 2 YCl3-catalyzed synthesis of benzothiazoles 3 from o-aminothiophenols 1a and aldehydes 2.a |
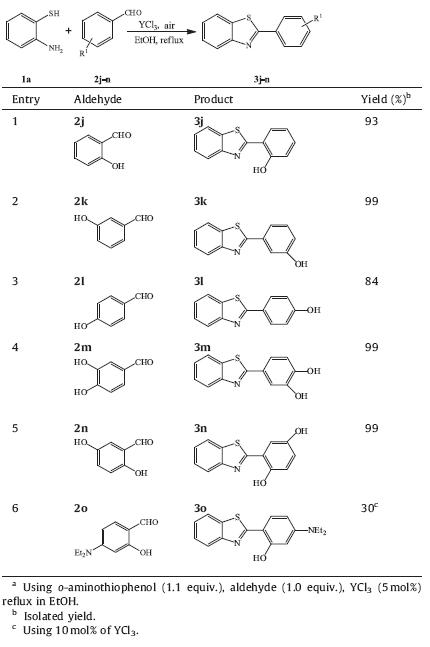
Nowadays development of novel method to synthesis antibacterial as well as antifungal agent is quite demanding and challenging for chemists and pharmacists. The present protocol has been applied to the synthesis of benzothiazoles 3j, 3k,3l,3m and 3n (Table 3,entries 1-5) which identified as potent β-glucuronidase inhibitors [27] in satisfactory yield. Compound 3o (Table 3,entry 6) which has strong antibacterial activity [28] was obtained in lower yield,because the reactant 2o with strong electron donor can be oxidized much easier than other reactants.
| Table 3 YCl3-catalyzed synthesis of bioactive benzothiazoles 3.a |
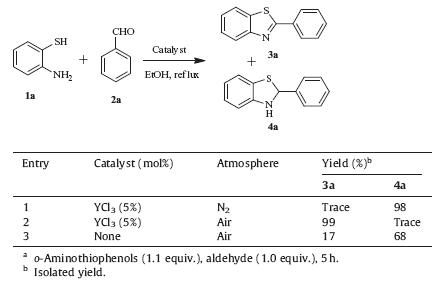
During the reaction procedure (Table 1,entry 1),2-phenylbenzothiazoline 4a which further dehydrated to form of 3a was detected by TLC. Obviously,oxidation is an indispensable process during this reaction. It was suspected that oxygen in air may act as an oxidant. In order to confirm our conjecture,three parallel experiments were performed and the results were summarized in Table 4.
| Table 4 Effect of atmosphere and catalyst in the formation of 3a.a |
Under nitrogen atmosphere,in the presence of YCl3,only a trace amount of benzothiazole 3a was detected whereas the intermediate product 4a was obtained in 98% yield (Table 4,entry 1). When the same reaction was carried out in air,the yield of 3a reached nearly 100% (Table 4,entry 2). It can therefore be deduced that oxygen in air as an oxidant promotes the transformation of 4a to target product 3a. In the absence of catalyst,compound 3a was produced only in 17% yield while 4a in 68% yield in air,indicating that the catalyst plays an important role not only in the condensation process but also in the oxidation.
According to the aforementioned observations,a tentative reaction mechanism is outlined in Scheme 2. The present reaction consists of Schiff base formation,intramolecular cyclization, dioxygen activation and heterocyclic compounds oxidation. Condensation of 1a and 2a generates the corresponding Schiff base A with YCl3 as an accelerant. The oxidative cyclization of Schiff base A could be envisioned to form heterocycle 4a which then undergoes YCl3-catalyzed aerobic oxidation to yield the desired product 3a.

|
Download:
|
| Scheme 2. Proposed reaction mechanism for the synthesis of benzothiazoles. | |
In conclusion,we developed an efficient synthetic protocol for the formation of benzothiazoles via YCl3-catalyzed aerobic oxidation reaction between 2-aminothiophenol and benzaldehyde. This method provides a green and practical synthetic approach to benzothiazoles that are important units of biologically active molecules. The usage of molecular oxygen in air renders this protocol very attractive and practical. Furthermore,our results demonstrated that yttrium catalyst plays a decisive role in aerobic oxidation. A detailed study on the scope of our synthetic methodology is ongoing in our laboratory.
AcknowledgmentWe gratefully acknowledge the National Natural Science Foundation of China (No. 20802052) for financial support. Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2014.10.017.
| [1] | M. Hudlicky, Oxidations in Organic Chemistry, American Chemical Society, Washington, D.C., 1990. |
| [2] | L. Chen, B.D. Li, Q.X. Xu, D.B. Liu, A silica gel supported cobalt(Ⅱ) Schiff base complex as efficient and recyclable heterogeneous catalyst for the selective aerobic oxidation of alkyl aromatics, Chin. Chem. Lett. 24 (2013) 849-852. |
| [3] | B.L. Mylari, E.R. Larson, T.A. Beyer, et al., Novel, potent aldose reductase inhibitors: 3,4-dihydro-4-oxo-3-[[5-(trifluoromethyl)-2-benzothiazolyl]methyl]-1-phthalazineacetic acid (zopolrestat) and congeners, J. Med. Chem. 34 (1991) 108-122. |
| [4] | (a) C.G. Mortimer, G. Wells, J.P. Crochard, et al., Antitumor benzothiazoles. 26.(1) 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610 NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines, J. Med. Chem. 49 (2006) 179-185;(b) S. Aiello, G. Wells, E.L. Stone, et al., Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648), J. Med. Chem. 51 (2008) 5135-5139. |
| [5] | J. Geng, M. Li, L. Wu, J. Ren, X. Qu, Liberation of copper from amyloid plaques: making a risk factor useful for Alzheimer's disease treatment, J. Med. Chem. 55 (2012) 9146-9155. |
| [6] | Y.H. Cho, C.Y. Lee, D.C. Ha, C.H. Cheon, Cyanide as a powerful catalyst for facile preparation of 2-substituted benzoxazoles via aerobic oxidation, Adv. Synth. Catal. 354 (2012) 2992-2996. |
| [7] | P. Bandyopadhyay, M. Sathe, G.K. Prasad, P. Sharma, M.P. Kaushik, Mesoporous mixed metal oxide nanocrystals: efficient and recyclable heterogeneous catalysts for the synthesis of 1,2-disubstituted benzimidazoles and 2-substituted benzothiazoles, J. Mol. Catal. A: Chem. 341 (2011) 77-82. |
| [8] | Y. Liao, H. Qi, S. Chen, et al., Efficient 2-aryl benzothiazole formation from aryl ketones and 2-aminobenzenethiols under metal-free conditions, Org. Lett. 14 (2012) 6004-6007. |
| [9] | N. Park, Y. Heo, M.R. Kumar, et al., Synthesis of benzothiazoles through coppercatalyzed one-pot three-component reactions with use of sodium hydrosulfide as a sulfur surrogate, Eur. J. Org. Chem. (2012) 1984-1993. |
| [10] | G.H. Sung, I.H. Lee, B.R. Kim, et al., Eco-friendly atom-economical synthesis of 2- substituted-benzo[d]thiazoles and 2-substituted-benzo[d]oxazoles using 2-acylpyridazin- 3(2H)-ones, Tetrahedron 69 (2013) 3530-3535. |
| [11] | Z. Yang, X. Chen, S. Wang, et al., Synthesis of 2-aryl benzothiazoles via K2S2O8- mediated oxidative condensation of benzothiazoles with aryl aldehydes, J. Org. Chem. 77 (2012) 7086-7091. |
| [12] | K. Bahrami, M.M. Khodaei, F. Naali, Mild and highly efficient method for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles, J. Org. Chem. 73 (2008) 6835-6837. |
| [13] | H.Y. Guo, J.C. Li, Y.L. Shang, A simple and efficient synthesis of 2-substituted benzothiazoles catalyzed by H2O2/HCl, Chin. Chem. Lett. 20 (2009) 1408-1410. |
| [14] | G. Evindar, R.A. Batey, Parallel synthesis of a library of benzoxazoles and benzothiazoles using ligand-accelerated copper-catalyzed cyclizations of ortho-halobenzanilides, J. Org. Chem. 71 (2006) 1802-1808. |
| [15] | K. Inamoto, C. Hasegawa, K. Hiroya, T. Doi, Palladium-catalyzed synthesis of 2- substituted benzothiazoles via a C-H functionalization/intramolecular C-S bond formation process, Org. Lett. 10 (2008) 5147-5150. |
| [16] | H. Hachiya, K. Hirano, T. Satoh, M. Miura, Nickel-catalyzed direct arylation of azoles with aryl bromides, Org. Lett. 11 (2009) 1737-1740. |
| [17] | M.G. Organ, M. Abdel-Hadi, S. Avola, et al., Biaryls made easy: PEPPSI and the Kumada-Tamao-Corriu reaction, Chem.: Eur. J. 13 (2007) 150-157. |
| [18] | M. Abdollahi-Alibeik, S. Poorirani, Perchloric acid-doped polyaniline as an efficient and reusable catalyst for the synthesis of 2-substituted benzothiazoles, Phosphorus Sulfur 184 (2009) 3182-3190. |
| [19] | T.G. Deligeorgiev, S. Kaloyanova, A. Vasilev, J.J. Vaquero, Novel green procedure for the synthesis of 2-arylbenzothiazoles under microwave irradiation in PEG 200 or PEG 400, Phosphorus Sulfur 185 (2010) 2292-2302. |
| [20] | T. Yamamoto, K. Muto, M. Komiyama, et al., Nickel-catalyzed C-H arylation of azoles with haloarenes: scope, mechanism, and applications to the synthesis of bioactive molecules, Chem.: Eur. J. 17 (2011) 10113-10122. |
| [21] | D. Crich, M. Patel, Radical dearomatization of arenes and heteroarenes, Tetrahedron 62 (2006) 7824-7837. |
| [22] | T. Itoh, T. Mase, A novel practical synthesis of benzothiazoles via Pd-catalyzed thiol cross-coupling, Org. Lett. 9 (2007) 3687-3689. |
| [23] | H. Sharghi, O. Asemani, Methanesulfonic acid/SiO2 as an efficient combination for the synthesis of 2-substituted aromatic and aliphatic benzothiazoles from carboxylic acids, Synth. Commun. 39 (2009) 860-867. |
| [24] | Y.M. Ha, J.Y. Park, Y.J. Park, et al., Synthesis and biological activity of hydroxy substituted phenyl-benzo[d]thiazole analogues for antityrosinase activity in B16 cells, Bioorg. Med. Chem. Lett. 21 (2011) 2445-2449. |
| [25] | C. Zhu, T. Akiyama, Benzothiazoline: highly efficient reducing agent for the enantioselective organocatalytic transfer hydrogenation of ketimines, Org. Lett. 11 (2009) 4180-4183. |
| [26] | H. Chikashita, M. Miyazaki, K. Itoh, 2-Phenylbenzothiazoline as a reducing agent in the conjugate reduction of α,β-unsaturated carbonyl compounds, Synthesis (1984) 308-310. |
| [27] | K.M. Khan, F. Rahim, S.A. Halim, et al., Synthesis of novel inhibitors of betaglucuronidase based on benzothiazole skeleton and study of their binding affinity by molecular docking, Bioorg. Med. Chem. 19 (2011) 4286-4294. |
| [28] | V.S. Padalkar, V.D. Gupta, K.R. Phatangare, et al., Indion 190 resin: efficient, environmentally friendly, and reusable catalyst for synthesis of benzimidazoles, benzoxazoles, and benzothiazoles, Green Chem. Lett. Rev. 5 (2012) 139-145. |