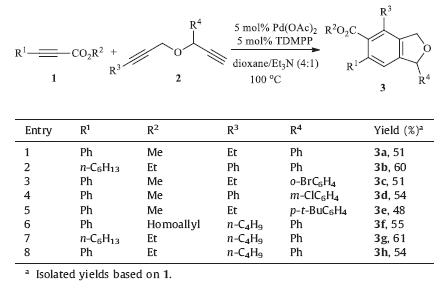
Phthalan (1,3-dihydrobenzofuran) represents an important class of heterocycles,which are frequently found in many biologically active natural products and pharmacologically important agents (Fig. 1) [1, 2]. For example,pestacin (I) was isolated from the microorganism pestalotiopsis microspore,and has been found to display potent antioxidant activity and moderate antifungal properties [3]. Citalopram (II) is a widely used antidepressant drug of the selective serotonin reuptake inhibitor (SSRI) class [4]. Phthalans have also received considerable interest in organic synthesisdue to theirunique andinteresting reactivity [5].Typically, the five-membered oxy-ring of phthalan can be opened reductively byan excess of lithiumin thepresence of a substoichometricamount of naphthalene to give dianionic intermediates,which have a wide use in organic synthesis [6].Moreover,through the activation of C-O bond of the heterocyclic unit by Lewis acids with subsequent C-N and C-C bond formation reactions,recent studies have demonstrated phthalans as good synthons for obtaining important unsaturated linear or arene compounds [7].
|
Download:
|
| Fig. 1. The structures of phthalan and two pharmaceutically important molecules. | |
On contrary to the importance in medicinal chemistry and organic synthesis,synthetic methods for phthalans are rather limited [1, 8, 9, 10, 11]. The most frequently employed strategy for their preparation is based on an intramolecular cyclization of the performed o-phthalyl alcohol or o-phthalyl halides,via either an electrophilic cyclization or a nucleophilic substitution [8]. It is apparent that these methods suffer from long procedure,troublesome preparation of the precursors and a lack of generality. The recent development of Ni,Co and Rh catalyzed [2 + 2 + 2] cyclotrimerization of oxy-tethered alkynes with carbon-carbon triple bonds have provided an attractive alternative [9]. However, the high-cost catalysts and specifically designed ligands were usually required,and mixtures of 1,3-dihydrobenzofuran isomers were often produced when asymmetric alkynes were used as substrates [9]. Other methods including the modified oxa-Pictet- Spengler reaction and hydrodeoxygenation or hydrodesulfurization of the performed phthalides were also reported,but only limited to the preparation of some phthalans such as hydroxyphthalans [10].
In this regard,we reported a highly efficient synthesis of fused phthalan derivatives from electron-deficient vinyl iodides and bispropargyl ethers in 2008,based on a cascade Sonogashira coupling,propargyl-allenyl isomerization and Diels-Alder cycloaddition reaction (CIRs) [11]. The methodology was also successfully extended to construct a variety of more complex phthalan derivatives via a two-fold coupling-isomerization sequence. However,the lack of efficient methods to generate the starting stereo-defined vinyl halides restricted the scope of the reaction and further applications. As a continued interest,quite recently we have realized a new atom-economic Pd-catalyzed addition- isomerization reaction sequence (AIRs) which employed very easily accessible (or commercially available) linear ynoates as the starting materials,leading to an easy access to a variety of 1,6,7,7atetrahydro- isobenzofurans in moderate to good yields [12]. In this paper,we wish to report the utility of AIRs to provide a facile synthesis of substituted phthalans directly from ynoate and bispropargyl ethers. 2. Experimental
All reactions were carried out under nitrogen atmosphere in Schlenk flask. Dry solvents were distilled prior to use: dioxane,THF and toluene were distilled from sodium-benzophenone; Et3N were distilled from CaH2. Petroleum ether (PE) refers to the fraction with boiling point in the range 60-90 °C. All 1H NMR and 13C NMR spectra were measured in CDCl3 with TMS as the internal standard. Chemical shifts were expressed in ppm and J values in Hz. Starting materials 1 were commercially available or prepared via a known procedure in literature [13]. Starting materials 2 were prepared according to known methods from the corresponding 1-aryl prop- 2-yn-1-ols and propargyl bromides [14].
General procedure for palladium-catalyzed sequential reactions of alkynoates with propargyl ethers: an oven-dried Schlenk tube containing a Teflon-coated stir bar was charged with Pd(OAc)2 (5.5 mg,5 mol%) and TDMPP (11.0 mg,5 mol%). The Schlenk tube was sealed and then evacuated and backfilled with N2 (3 cycles). 1,4-Dioxane (1 mL) was then injected under N2. After stirring at room temperature for 15 min,a solution of ynoate 1 (0.5 mmol) and propargyl ether 2 (0.75 mmol) in 0.5 mL of 1,4-dioxane and 0.5 mL of Et3N was subsequently injected. The reaction mixture was subjected to a preheated oil bath at 100 °C for 2 h,and further kept stirring at room temperature overnight. After the reaction was complete (monitored by TLC),removal of the solvent in vacuo left a slurry residue,which was purified by flash chromatography on silica to afford 3.
Methyl 4-ethyl-1,6-diphenyl-1,3-dihydroisobenzofuran-5-carboxylate (3a): Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 2949, 1728,1453,1257,1160,701. 1H NMR (CDCl3,400 MHz): δ 7.37- 7.26 (m,10H),6.91 (s,1H),6.23 (s,1H),5.44 (d,1H,J1,J2 = 12.4 Hz), 5.30 (dd,1H,J1,J2 = 12.4 Hz),3.57 (s,3H),2.73-2.63 (m,2H),1.27 (t, 3H,J = 7.3 Hz). 13C NMR (CDCl3,100 MHz): δ 170.1,143.5,141.5, 140.7,140.6,137.2,135.3,132.4,128.6,128.2,128.1,127.4,126.9, 121.3,86.5,72.3,51.9,24.9,14.7. HRMS (EI-TOF) (m/z): calcd. for C24H22O3 [M+] 358.1569; found 358.1562.
Ethyl 6-hexyl-1,4-diphenyl-1,3-dihydroisobenzofuran-5-carboxylate (3b): Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 2948,1729,1454,1257,1158,701. 1H NMR (CDCl3,400 MHz): δ 7.42-7.25 (m,10H),6.87 (s,1H),6.15 (s,1H),5.12 (d,1H,J = 2.8 Hz), 5.06 (d,1H,J = 2.8 Hz),3.98 (q,2H,J = 7.2 Hz),2.65-2.61 (m,2H), 1.57-1.53 (m,2H),1.32-1.26 (m,6H),0.91 (t,1H,J = 7.2 Hz),0.85 (t,1H,J = 6.8 Hz). 13C NMR (CDCl3,100 MHz): δ 169.2,143.7,142.6, 140.8,138.2,135.8,134.5,134.0,133.3,129.9,128.4,128.3,128.2, 127.8,127.1,125.1,122.0,85.8,73.0,60.9,33.7,31.5,29.2,22.5, 14.0,13.6. HRMS (EI-TOF) (m/z): calcd. for C29H32O3 [M+] 428.2351; found 428.2356.
Methyl 1-(2-bromophenyl)-4-ethyl-6-phenyl-1,3-dihydroisobenzofuran- 5-carboxylate (3c): Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 2950,1728,1436,1257,1161,1026,760,701. 1H NMR (CDCl3,400 MHz): δ 7.58 (d,J = 7.8 Hz,1H),7.37-7.26 (m, 7H),7.17-7.08 (m,2H),6.72 (s,1H),5.44 (dd,1H,J1,J2 = 12.4, 2.8 Hz),5.30 (dd,1H,J1,J2 = 12.4,1.8 Hz),3.55 (s,3H),2.73-2.58 (m,2H),1.24 (t,3H,J = 7.8 Hz). 13C NMR (CDCl3,100 MHz): δ 170.1, 142.8,141.0,140.8,140.6,137.2,135.4,132.9,132.6,129.6,128.9, 128.3,128.2,127.9,127.4,122.3,121.4,85.1,72.6,52.0,25.0,14.8. HRMS (EI-TOF) (m/z): calcd. for C24H21BrO3 [M+] 436.0674; found 436.0679.
Methyl 1-(3-chlorophenyl)-4,6-diphenyl-1,3-dihydroisobenzofuran- 5-carboxylate (3d): Slurry solid (from PE/EtOAc). IR (KBr, cm-1): v 2947,1733,1436,1284,1128,702. 1H NMR (CDCl3, 400 MHz): δ 7.45-7.24 (m,14H),7.03 (s,1H),6.22 (s,1H),5.22 (dd, 1H,J1,J2 = 12.8,2.7 Hz),5.11 (dd,1H,J1,J2 = 12.8,1.8 Hz),3.31 (s, 3H). 13C NMR (CDCl3,100 MHz): δ 169.3,143.5,142.9,140.7,140.1, 137.6,137.3,134.6,134.3,133.2,130.0,128.5,128.4,128.33, 128.28,128.1,128.0,127.7,127.0,125.0,122.6,85.9,73.1,51.8. HRMS (EI-TOF) (m/z): calcd. for C28H21ClO3 [M+] 440.1179; found 440.1173.
Methyl 1-(4-tert-butylphenyl)-4-ethyl-6-phenyl-1,3-dihydroisobenzofuran- 5-carboxylate (3e): Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 2964,1730,1454,1258,703. 1H NMR (CDCl3, 400 MHz): δ 7.37-7.24 (m,9H),6.92 (s,1H),6.20 (s,1H),5.39 (dd, 1H,J1,J2 = 12.4,2.3 Hz),5.25 (dd,1H,J1,J2 = 12.4,1.4 Hz),3.55 (s, 3H),2.73-2.58 (m,2H),1.29-1.22 (m,12H). 13C NMR (CDCl3, 100 MHz): δ 170.1,151.2,143.5,140.7,140.6,138.4,137.4,135.3, 132.3,128.22,128.17,127.4,126.7,125.5,121.4,86.4,72.1,51.9, 34.5,31.3,24.9,14.7. HRMS (EI-TOF) (m/z): calcd. for C28H30O3 [M+] 414.2195; found 414.2189.
But-3-enyl 4-butyl-1,6-diphenyl-1,3-dihydroisobenzofuran-5- carboxylate (3f): Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 3062,3030,2956,1732,1455,1243,699. 1H NMR (CDCl3, 400 MHz): δ 7.35-7.26 (m,10H),6.87 (s,1H),6.20 (s,1H),5.52- 5.39 (m,2H),5.28-5.24 (m,1H),4.96-4.92 (m,2H),4.02-3.98 (m, 2H),2.70-2.58 (m,2H),2.08-2.03 (m,2H),1.63-1.55 (m,3H), 1.46-1.37 (m,2H),0.95 (t,3H,J = 7.2 Hz). 13C NMR (CDCl3, 100 MHz): δ 169.6,143.3,141.6,140.8,140.7,137.5,134.2,133.7, 132.6,128.6,128.3,128.2,127.4,127.0,121.3,117.0,86.6,72.5, 64.3,32.7,32.4,31.4,23.0,13.9. HRMS (EI-TOF) (m/z): calcd. for C29H30O3 [M+] 426.2195; found 426.2196.
Ethyl 4-butyl-6-hexyl-1-phenyl-1,3-dihydroisobenzofuran-5- carboxylate (3g): Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 2945,1728,1453,1255,1158,700. 1H NMR (CDCl3,400 MHz): d 7.38-7.30 (m,5 H),6.72 (s,1H),6.13 (s,1H),5.31 (dd,1H,J1, J2 = 12.0,2.4 Hz),5.17 (dd,1H,J1,J2 = 12.0,2.4 Hz),4.39 (q,2H, J = 7.2 Hz),2.55-2.49 (m,4H),1.58-1.47 (m,4H),1.39 (t,3H, J = 7.2 Hz),1.32-1.25 (m,8H),0.94 (t,1H,J = 7.2 Hz),0.85 (t,1H, J = 6.8 Hz). 13C NMR (CDCl3,100 MHz): δ 170.1,143.0,141.8,140.1, 135.9,132.2,128.5,128.1,127.1,120.7,86.6,72.4,61.0,33.9,32.5, 31.60,31.56,31.46,29.2,23.0,22.5,14.2,14.0,13.8. HRMS (EI-TOF) (m/z): calcd. for C27H36O3 [M+] 408.2664; found 408.2661.
Ethyl 4-butyl-1,6-diphenyl-1,3-dihydroisobenzofuran-5-carboxylate (3h): Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 2957,2871,1732,1456,1253,700. 1H NMR (CDCl3,400 MHz): d 7.35-7.25 (m,10H),6.87 (s,1H),6.20 (s,1H),5.40 (dd,1H,J1, J2 = 12.0,2.4 Hz),5.26 (dd,1H,J1,J2 = 12.0,1.6 Hz),4.02 (q,2H, J = 7.2 Hz),2.69-2.60 (m,2H),1.46-1.39 (m,2H),0.97-0.91 (m, 6H). 13C NMR (CDCl3,100 MHz): δ 169.5,143.3,141.6,140.8,140.7, 137.5,134.1,132.8,128.6,128.3,128.2,128.1,127.3,126.9,121.2, 86.6,72.5,61.0,32.6,31.4,23.0,13.9,13.6. HRMS (EI-TOF) (m/z): calcd. for C27H28O3 [M+] 400.2038; found 400.2035.
3i: Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 2953,2867, 1721,1466,1253,1162,1027,699. 1H NMR (CDCl3,400 MHz): d 7.27-7.19 (m,10H),6.78 (s,1H),6.12 (s,1H),5.35-5.31 (m,1H), 5.20-5.17 (m,2H),4.52-4.42 (m,1H),2.61-2.57 (m,2H),1.93-0.78 (m,47H),0.58 (s,3H). 13C NMR (CDCl3,100 MHz): δ 168.9,143.2, 141.7,140.9,140.7,139.5,137.5,134.0,133.1,128.64,128.55, 128.25,128.20,127.4,127.0,122.68,122.66,121.3,86.7,75.0,72.6, 56.7,56.1,50.0,42.3,39.7,39.5,37.5,37.4,36.9,36.6,36.2,35.8, 32.7,31.9,31.8,31.4,28.2,28.0,27.3,27.2,24.3,23.8,23.1,22.9, 22.6,21.0,19.2,18.7,14.0,11.9. HRMS (EI-TOF) (m/z): calcd. for C52H68O3 [M+] 740.5168; found 740.5161.
Ethyl 1,4,6-triphenyl-3,5-dihydroisobenzofuran-5-carboxylate (4): Slurry solid (from PE/EtOAc). IR (KBr,cm-1): v 3060,2979, 1730,1494,1450,1271,1114,758,701. 1H NMR (CDCl3,400 MHz): d 7.73 (d,2H,J = 7.6 Hz),7.56 (d,2H,J = 8.0 Hz),7.45-7.26 (m,11H), 7.13 (s,1H),5.47 (dd,1H,J1,J2 = 16.0,3.2 Hz),5.29 (s,1H),5.14 (dd, 1H,J1,J2 = 16.0,3.2 Hz),3.89-3.81 (m,2H),0.80 (t,3H,J = 7.2 Hz). 13C NMR (CDCl3,100 MHz): δ 171.1,156.7,139.8,139.2,138.7, 134.3,130.9,129.2,128.6,128.4,127.6,127.0,126.83,126.76, 126.1,118.2,117.9,113.7,72.4,60.8,52.3,13.7. HRMS (EI-TOF) (m/ z): calcd. for C29H24O3 [M+] 420.1725; found 420.1721. 3. Results and discussion
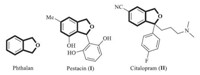
The reaction of (1a) and (2a) was conducted under the conditions similar to previously reported by us [12],affording the desired product in 51% yield (Table 1,entry 1). The reaction also proceeds smoothly for an alkylpropiolate (e.g.,ethyl non-2- ynoate),affording the desired product 3b in 60% yield (Table 1, entry 2). The expected product 3f was also obtained in 55% yield when but-3-enyl 3-phenylpropiolate was employed (Table 1,entry 6),indicating the tolerance of the double bond appended in ynoate. For the bispropargyl ethers,R3 could be an alkyl group (e.g.,ethyl, butyl group) or a phenyl group,and R4 could be substituted phenyl group with either electron-donating or electron-withdrawing group (Table 1,entries 3-5). Noteworthy,a bromide functionality appended on the aryl ring is also compatible with the current palladium catalyst (Table 1,entry 3). The products 3a-3h were all characterized by spectroscopy techniques including 1H NMR,13C NMR,IR and HRMS,and the results are summarized in Table 1.
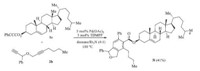
As for the application,the present reaction could be employed as a promising approach for modification of important bioactive molecules or drug candidates. As shown in Scheme 1,when substrate 1c derived from cholesterol was employed to react with the propagyl ether 2b,a complex natural-product-like molecule 3i was obtained in 41% yield.
| Table 1 Pd-catalyzed tandem reaction of ynoates with propargyl ethers to produce 1,3- dihydroisobenofurans 3. |
|
Download:
|
| Scheme 1. Synthetic application. | |
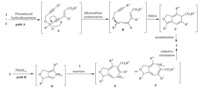
Mechanistically,two plausible reaction pathways were considered (Scheme 2). The first one starts from the intermolecular addition of a terminal alkyne 2 to the electron-deficient alkyne 1 to produce an ene-enyne species A. The combination of Pd(OAc)2 and TDMPP serves as the catalyst to implement the addition reaction [15]. Subsequently,the organic base Et3N promotes a propargyl- allenyl isomerization process,yielding an ene-vinylallene intermediate B. Then an intramolecular Diels-Alder cycloaddition reaction occurs to give rise to the intermediate C. Aromatization of C via C55C bond migration finally affords the product 3 (Scheme 2, path). Alternatively,another reaction pathway involving cyclopalladation of 2 followed by insertion of 1 into the palladacycle D and subsequent reductive elimination was also possible (Scheme 2,path B). However,a control experiment revealed that the reaction of 1b and 2c at 80 °C for 1 h produced the cyclic triene compound 4,a product similar to the intermediate C (Scheme 3). The identification of compound 4 thus confirmed that path A should be the most likely mechanism.
|
Download:
|
| Scheme 2. The plausible mechanism for the formation of 3. | |

|
Download:
|
| Scheme 3. Identification of the reaction intermediate 4. | |
In conclusion,we have developed a tandem hydroalkynylation, alkyne-allene isomerization,Diels-Alder cycloaddition and aromatization sequence to provide a facile synthesis of phthalan derivatives in moderate yields. The reaction carries the advantages of easily available starting materials and high efficiency. A plausible mechanism was also proposed to rationalize the reaction with evidence of the determination of reaction intermediates.
AcknowledgmentsThe financial support from the National Natural Science Foundation of China (No. 21302095),Research Fund for the Doctoral Program of Higher Education of China (No. 20133221120003),Jiangsu Provincial NSFC (No. BK20130924) and Foundation of Jiangsu Educational Committee of China (No. 13KJB150019) is fully acknowledged. This work was also partially supported by the Project of Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). R.S. thanks Prof. L. Wu of Zhejiang University for the help on HRMS analysis.
| [1] | R. Karmakar, P. Pahari, D. Mal, Phthalides and phthalans: synthetic methodologies and their applications in the total synthesis, Chem. Rev. 114 (2014) 6213-6284. |
| [2] | (a) J.F. Debernardis, D.L. Arendsen, J.J. Kyncl, D.J. Kerkman, Conformationally defined adrenergic agents. 4. 1-(aminomethyl)phthalans: synthesis and pharmacological consequences of the phthalan ring oxygen atom, J. Med. Chem. 30 (1987) 178-184;(b) D.S. Kim, K.K. Kang, K.S. Lee, et al., Synthesis and biological properties of new 5-cyano-1,1-disubstituted phthalans for the treatment of premature ejaculation, Bull. Korean Chem. Soc. 29 (2008) 1946-1950;(c) D.A. Oparin, Z.V. Motylevich, Synthesis and antitumor activity of monomethinecyanine dyes of the phthalan series with nuclei of phthalazine, benzo-1,2- dithiol, and thiophthalan, Pharm. Chem. J. 28 (1994) 233-235. |
| [3] | J.K. Harper, A.M. Arif, E.J. Ford, et al., Pestacin: a 1,3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities, Tetrahedron 59 (2003) 2471-2476. |
| [4] | B.G. Pollock, Citalopram: a comprehensive review, Exp. Opin. Pharmacother. 2 (2001) 681-698. |
| [5] | (a) D. Garcia, F. Foubelo, M. Yus, Regioselective reductive opening of substituted phthalans: synthetic applications, Tetrahedron 64 (2008) 4275-4286;(b) A. Lifshitz, A. Suslensky, C. Tamburu, Thermal reactions of isodihydrobenzofuran: experimental results and computer modeling, J. Phys. Chem. A. 105 (2001) 3148-3157;(c) A.E. Dorigo, K.N. Houk, T. Cohen, Unexpected regioselectivity in the reductive cleavage of epoxides - a theoretical rationalization, J. Am. Chem. Soc. 111 (1989) 8976-8978. |
| [6] | (a) U. Azzena, S. Demartis, G. Melloni, Electron-transfer-induced reductive cleavage of phthalans: reactivity and synthetic applications, J. Org. Chem. 61 (1996) 4913-4919;(b) L.R. Falvello, F. Foubelo, T. Soler, M. Yus, Structural modification of steroids through functionalized organolithium compounds, Tetrahedron: Asymmetry 11 (2000) 2063-2066;(c) F. Foubelo, T. Soler, M. Yus, Synthesis of functionalized enantiopure steroids from estrone and cholestanone through organolithium intermediates, Tetrahedron: Asymmetry 12 (2001) 801-810;(d) M. Yus, J. Gomis, ZnBr2/CuCN-promoted, highly regioselective S(N)2' reactions of some functionalized organolithium compounds with allylic and propargylic halides, Eur. J. Org. Chem. (2003) 2043-2048;(e) M. Yus, J. Gomis, Negishi cross-coupling with functionalised organozinc compounds prepared by lithium-zinc transmetallation, Tetrahedron Lett. 42 (2001) 5721-5724;(f) T. Soler, A. Bachki, L.R. Falvello, F. Foubelo, M. Yus, Structural modification of carbohydrates via functionalised organolithium intermediates: EPC preparation of branched-chain functionalised sugars, Tetrahedron: Asymmetry 11 (2000) 493-517. |
| [7] | (a) Y. Sawama, K. Shibata, Y. Sawama, et al., Iron-catalyzed ring-opening azidation and allylation of O-heterocycles, Org. Lett. 15 (2013) 5280-5285;(b) Y. Sawama, Y. Sawama, N. Krause, Highly regioselective gold-catalyzed ringopening allylation and azidation of dihydrofurans, Org. Lett. 11 (2009) 5034- 5037. |
| [8] | (a) B. Panda, T.K. Sarkar, A one-pot tandem oxidation-reduction protocol for the synthesis of cyclic ethers from their diols, Tetrahedron Lett. 49 (2008) 6701- 6703;(b) K. Kobayashi, K. Shikata, S. Fukamachi, H. Konishi, A facile synthesis of 1,3- dihydroisobenzofurans using iodocylization of 2-vinylbenzyl alcohols, Heterocycles 75 (2008) 599-609;(c) V. Capriati, S. Florio, R. Luisi, B. Musio, Directed ortho lithiation of N-alkylphenylaziridines, Org. Lett. 7 (2005) 3749-3752;(d) V. Capriati, S. Florio, R. Luisi, F.M. Perna, A. Salomone, Synthesis of 1,3- dihydrobenzo[c]furans from ortho-lithiated aryloxiranes, J. Org. Chem. 71 (2006) 3984-3987;(e) M. Rottländer, P. Knochel, Synthesis of 2,4-disubstituted 2,5-dihydrofurans and 1-substituted 1,3-dihydroisobenofurans via an iodine-magnesium exchange reaction, J. Comb. Chem. 1 (1999) 181-183. |
| [9] | P.R. Chopade, J. Louie, [2 + 2 + 2] Cycloaddition reactions catalyzed by transition metal complexes, Adv. Synth. Catal. 348 (2006) 2307-2327. |
| [10] | (a) M. Guiso, A. Betrow, C. Marra, The oxa-Pictet-Spengler reaction: a highlight on the different efficiency between isochroman and phthalan or homoisochroman derivatives synthesis, Eur. J. Org. Chem. (2008) 1967-1976;(b) R. Karmakar, P. Pahari, D. Mal, A synthetic route to 1,3-dihydroisobenzofuran natural products: the synthesis of methyl ethers of pestacin, Tetrahedron Lett. 50 (2009) 4042-4045;(c) P. Wang, R. Zhang, J. Cai, J.Q. Chen, M. Ji, Efficient synthesis of functionalized 1,3-dihydroisobenzofurans from salicylaldehydes: application to the synthesis of escitalopram, Chin. Chem. Lett. 25 (2014) 549-552;(d) Y.H. Liu, T.M. Fu, C.Y. Ou, W.L. Fan, G.P. Peng, Improved preparation of (1S, 30R,40S,50S,60R)-5-chloro-6-[(4-ethylphenyl)methyl]-3',4',5',6'-tetrahydro-6'- (hydroxymethyl)-spiro[isobenzofuran-1(3H),2'-[2H]pyran]-3',4',5'-triol, Chin. Chem. Lett. 24 (2013) 131-133. |
| [11] | R. Shen, X. Huang, L. Chen, A facile and efficient synthesis of dihydroisobenzofuran derivatives via tandem palladium-catalyzed coupling, propargyl-allenyl rearrangement,[4 + 2] cycloaddition and aromatization reaction, Adv. Synth. Catal. 350 (2008) 2865-2870. |
| [12] | R. Shen, K. Chen, Q. Deng, J. Yang, L. Zhang, Highly stereoselective generation of complex oxy-bicyclic scaffolds via an atom-economic Pd(Ⅱ)-catalyzed hydroalkynylation, isomerization and Diels-Alder cycloaddition sequence, Org. Lett. 16 (2014) 1208-1211. |
| [13] | B. Neises, W. Steglich, Simple method for the esterification of carboxylic acids, Angew. Chem. Int. Ed. 17 (1978) 522-524. |
| [14] | T.E. Nielsen, S. Le Quement, D. Tanner, Palladium-catalyzed silastannation of secondary propargylic alcohols and their derivatives, Synthesis 9 (2004) 1381-1390. |
| [15] | B.M. Trost, M.T. Sorum, C. Chan, A.E. Harms, G. Rühter, Palladium-catalyzed additions of terminal alkynes to acceptor alkynes, J. Am. Chem. Soc. 119 (1997) 698-708. |