Given the importance of pyridine N-oxide derivatives in medical chemistry as well as their easy deoxygenation to the more important pyridine derivatives,methods for the synthesis of pyridine N-oxide derivatives continue to attract considerable interest in organic synthesis [1,2,3,4,5]. Fagnou et al. reported the palladium-catalyzed regioselective direct arylation of pyridineNoxides with aryl bromides,which was used for the preparation of substituted pyridines and other heterocycles [6,7]. Recently,the C- H bond activation approach using pyridine N-oxides was served as an attractive platform for the 2-functionalization of pyridine species [8,9,10,11,12,13,14]. However,these methods require harsh conditions and long reaction time.
In 1999,Lohse et al.reported a catalytic system of DME/H2O/ K2CO3/5%Pd(PPh3)4 for the Suzuki cross-coupling reaction between 2- or 4-chloropyridine N-oxide and arylboronic acid, affording 65%-70% isolated yield of the desired products,which provided an alternative for the synthesis of aryl-substituted pyridine N-oxide derivatives [15]. To the best of our knowledge, this is the only example of the arylation of pyridine N-oxidesvia the Suzuki reaction. The palladium-catalyzed Suzuki reaction of aryl halides with arylboronic acids is one of the most versatile and powerful tools to form biaryls,which has been extensively used in the synthesis of pharmaceuticals,herbicides,natural products and advanced functional materials [16,17]. However,N-heteroaryl halides are generally inactive substrates for the Suzuki reaction due to the potential coordination of the nitrogen to the active palladium species [18]. So far,a lot of achievements have been made to activate these substrates,including the development of ligand-promoted protocols as Lohse et al.did [15]. Our group has reported ligand-free approaches to activate 2-bromopyridine derivatives successfully in ethylene glycol or EtOH/H2O [19,20]. At the moment,we are interested in activating 2-bromopyridine derivatives for the palladium-catalyzed Suzuki reaction in water without any additional ligand.
The use of water as sole reaction mediumhas several advantages, such as abundance,non-toxic,non-corrosiveness and improved safety [21,22,23,24,25]. Developing organic reaction in pure water is one of the latest challenges for modern chemists. Herein,we report an efficient method for the Pd(OAc)2-catalyzed Suzuki reaction of 2- or 3-bromopyridine N-oxides in pure water without any ligand. 2. Experimental
Aryl halides and arylboronic acids were purchased from Alfa Aesar. Other chemicals were obtained commercially and used without purification.
1H NMR spectra were recorded on a Bruker AvanceII 400 spectrometer using TMS as internal standard. 13C NMR spectra were recorded at 100 MHz using TMS as internal standard. All products were isolated by short chromatography on a silica gel (200-300 mesh) column using petroleum ether (60- 90°C),unless otherwise noted.
For this study,pyridine N-oxides were synthesized according to the following general procedures: pyridyl halides (1.0 equiv.) and m-chloroperoxybenzoic acid (1.1 equiv.) are dissolved in dry methylene dichloride. The reaction is allowed to stir at room temperature overnight. The solvent is then evaporated under reduced pressure and the crude reaction mixture is purified by column chromatography on silica gel with CH2Cl2 or CH2Cl2/MeOH mixtures.
All Suzuki reactions were carried out under air atmosphere. A mixture of bromopyridine N-oxide (0.5 mmol),arylboronic acid (0.75 mmol),(i-Pr)2NH (1.0 mmol),Pd(OAc)2 (0.25 mol%),H2O (1.0 mL) was allowed to react at 100°C. The reaction mixture was added to brine (10 mL) and extracted with CH2Cl2(3×10 mL). The solvent was concentrated under vacuum and the product was isolated by short chromatography on a silica gel (200-300 mesh) column.
The characterization and spectra of all products are available in the Supporting information. 3. Results and discussion
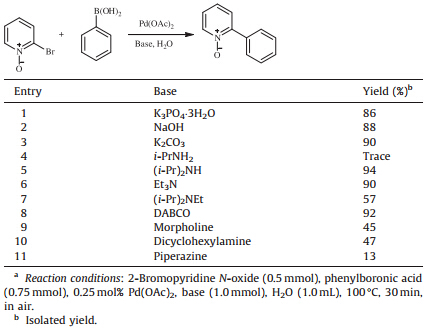
Initially,the cross-coupling of 2-bromopyridine N-oxide with phenylboronic acid was chosen as a model reaction for screening bases. The experimental data presented in Table 1.
|
Table 1 Effects of bases on the Suzuki cross-coupling reaction of 2-bromopyridine N-oxide with phenylboronic acid.a |
Water-soluble inorganic bases gave comparative yields (Table 1,entries 1-3),which were better than the results of cross-coupling of 4-bromoanisole with arylboronic acid as we reported [26]. The reason for this is supposed due to the highly solubility of 2-bromopyridine N-oxide in water. Several organic bases were examined and (i-Pr)2 NH,Et3N,DABCO (1,4-diazabicyclo[2.2.2]octane) (Table 1,entries 5,6 and 8) provided relatively high yields compared to the other bases. The results show that (iPr)2NH (Table 1,entry 5) is the best choice,which provided a 94% isolated yield in 30 min.
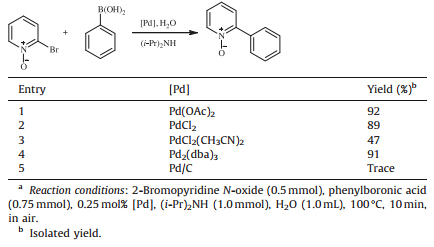
The palladium source employed in the Suzuki reaction is also important to the catalytic efficiency. As shown in Table 2,the palladium sources have dramatic effects on the reaction activity under the present conditions. The results indicated that Pd(OAc)2, Pd2(dba)3 and PdCl2 exhibited high catalytic activity. PdCl2(CH3CN)2 gave relatively low yields. Pd/C just provided trace yield in 10 min. It is supposed that PdCl2(CH3CN)2 and Pd/C might have a long activation period in the present catalytic system. We chose Pd(OAc)2 as the catalyst for the following study.
|
Table 2 Effects of palladium species on the cross-coupling of 2-bromopyridine N-oxide with phenylboronic acid.a |
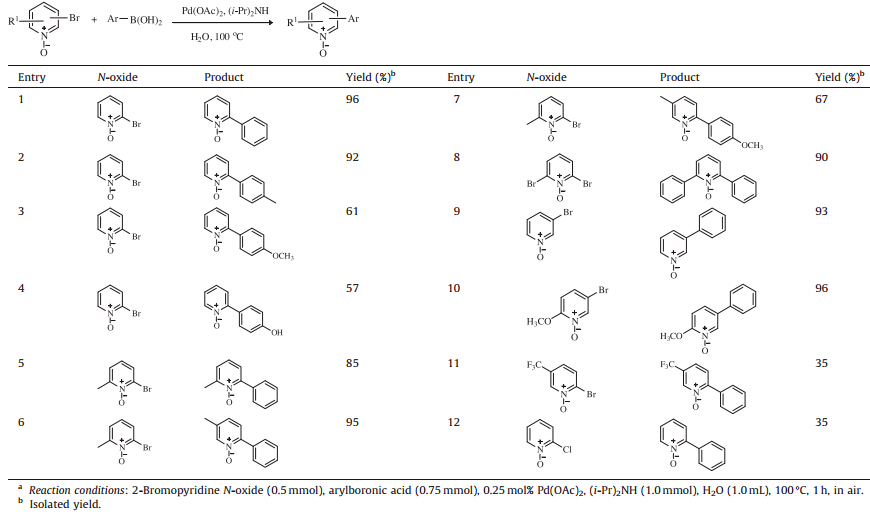
We further investigated the scope of direct arylation of bromopyridine N-oxides under the conditions of 0.25 mol% Pd(OAc)2 and two equivalents of (i-Pr)2 NH at 100°C in water.
The results are shown in Table 3. The cross-coupling of 2-bromopyridine N-oxide with phenylboronic acid or 4-methylphenlyboronic acid could afford above 92% isolated yields in 1 h (Table 3,entries 1 and 2),which were more efficient than the previous report that 2-tolylpyridine N-oxide was obtained in 91% isolated yield overnight from the reaction between pyridine Noxide and 4-bromotoluene [7]. The reaction between 2-bromopyridine N-oxide and 4-methoxylphenylboronic acid or 4-hydroxyphenylboronic acid provided a lower isolated yield of 61% and 57% in 1 h (Table 3,entries 3 and 4). Methylpyridine N-oxides could be activated by this catalytic system,affording 67%-95% isolated yield (Table 3,entries 5-7). The cross-coupling of 2,6-dibromopyridine N-oxide with phenylboronic acid performed efficiently, providing a 90% isolated yield in 1 h (Table 3,entry 8). The arylation of 3-bromopyridine N-oxide or 2-methoxyl-5-bromopyridine N-oxide proceeded very quickly with the yields of 93% and 96%,respectively (Table 3,entries 9 and 10). However,the crosscoupling of 2-bromo-5-(trifluoromethyl)-pyridine N-oxide or 2-chloropyridine N-oxide with phenylboronic acid was carried out slowly than that of 2-bromopyridine N-oxide (Table 3,entries 11 and 12).
|
Table 3 The Suzuki reaction of bromopyridine N-oxides with arylboronic acids.a |
In summary,we have developed an efficient protocol for the arylation of pyridine N-oxides with arylboronic acid. In view of the importance of pyridine N-oxide derivatives in medical chemistry, the Suzuki reaction will find a broad use in organic synthesis. Extending the scope of Suzuki reaction to other heterocyclicNoxides is in progress in our laboratory.
Acknowledgment
The authors thank the financial support from the National Natural Science Foundation of China (No. 21276043).
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet. 2014.09.019.
| [1] | M.H. Mosslemin, N. Shams, H. Esteghamat, H. Anaraki-Ardakani, An efficient synthesis of functionalized tetrahydro-1H-indeno[2,1-c]pyridine derivatives by a PPh3-promoted condensation reaction between acetylene esters and 1,3-dioxo- N-aryl-2,3-dihydro-1H-indene-2-carboxamides, Chin. Chem. Lett. 24 (2013) 1095-1098. |
| [2] | W.P. Mai, J.W. Yuan, Z.C. Li, G.C. Sun, L.B. Qu, Silver-catalyzed 2-pyridyl arylation of pyridine N-oxides with arylboronic acids at room temperature, Synlett 23 (2012) 145-149. |
| [3] | J. Balzarini, E. Keyaerts, L. Vijgen, et al., Inhibition of feline (FIPV) and human (SARS) corona virus by semisynthetic derivatives of glycopeptide antibiotics, Antivir. Res. 72 (2006) 20-33. |
| [4] | J.A. Pool, B.L. Scott, J.L. Kiplinger, A new mode of reactivity for pyridine N-oxide: C- H activation with uranium(IV) and thorium(IV) bis(alkyl) complexes, J. Am. Chem. Soc. 127 (2005) 1338-1339. |
| [5] | M. Ramakrishna Prasad, G. Kamalakar, G. Madhavi, S.J. Kulkarni, K.V. Raghavan, An efficient synthesis of heterocyclic-oxides over molecular sieve catalysts, Chem. Commun. (2000) 1577-1578. |
| [6] | J.P. Leclerc, K. Fagnou, Palladium-catalyzed cross-coupling reactions of diazine Noxides with aryl chlorides, bromides, and iodides, Angew. Chem. Int. Ed. 45 (2006) 7781-7786. |
| [7] | L.C. Campeau, S. Rousseaux, K. Fagnou, A solution to the 2-pyridyl organometallic cross-coupling problem: regioselective catalytic direct arylation of pyridine Noxides, J. Am. Chem. Soc. 127 (2005) 18020-18021. |
| [8] | S. Zhang, L.Y. Liao, F. Zhang, X.F. Duan, Arylation, alkenylation, and alkylation of 2- halopyridine N-oxides with Grignard reagents: a solution to the problem of C2/C6 regioselective functionalization of pyridine derivatives, J. Org. Chem. 78 (2013) 2720-2725. |
| [9] | J.T. Myers, J.M. Hanna Jr., Palladium-catalyzed direct arylation of pyridine Noxide with 2-bromoacetanilides. Synthesis of benzisoxazolo[2,3-a]pyridinium tetrafluoroborates, Tetrahedron Lett. 53 (2012) 612-615. |
| [10] | L. Ackermann, S. Fenner, Direct arylations of electron-deficient (hetero)arenes with aryl or alkenyl tosylates and mesylates, Chem. Commun. 47 (2011) 430- 432. |
| [11] | L.C. Campeau, K. Fagnou, Synthesis of 2-aryl pyridines by palladium-catalyzed direct arylation of pyridine N-oxides, Org. Synth. 88 (2011) 22-32. |
| [12] | L.C. Campeau, M. Bertrand-Laperle, J.P. Leclerc, et al., C2, C5, and C4 Azole N-oxide direct arylation including room-temperature reactions, J. Am. Chem. Soc. 130 (2008) 3276-3277. |
| [13] | L.C. Campeau, D.J. Schipper, K. Fagnou, Site-selective sp2 and benzylic sp3 palladium-catalyzed direct arylation, J. Am. Chem. Soc. 130 (2008) 3266- 3267. |
| [14] | S.H. Cho, S.J. Hwang, S. Chang, Palladium-catalyzed C-H functionalization of pyridine N-oxides: highly selective alkenylation and direct arylation with unactivated arenes, J. Am. Chem. Soc. 130 (2008) 9254-9256. |
| [15] | O. Lohse, P. Thevenin, E. Waldvogel, The palladium catalysed Suzuki coupling of 2- and 4-chloropyridines, Synlett (1999) 45-48. |
| [16] | A. Suzuki, Cross-coupling reactions of organoboranes: an easy way to construct CC bonds (Nobel Lecture), Angew. Chem. Int. Ed. 50 (2011) 6722-6737. |
| [17] | N. Miyaura, T. Yanagi, A. Suzuki, The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases, Synth. Commun. 11 (1981) 513-519. |
| [18] | T. Itoh, T. Mase, Direct synthesis of hetero-biaryl compounds containing an unprotected NH2 group via Suzuki-Miyaura reaction, Tetrahedron Lett. 46 (2005) 3573-3577. |
| [19] | X.F. Rao, C. Liu, Y. Xing, et al., Oxygen-promoted palladium-on-carbon-catalyzed ligand-free Suzuki reaction for the synthesis of heterobiaryls in aqueous media, Asian J. Org. Chem. 2 (2013) 514-518. |
| [20] | C. Liu, N. Han, X.X. Song, J.S. Qiu, A general and highly efficient method for the construction of aryl-substituted N-heteroarenes, Eur. J. Org. Chem. 2010 (2010) 5548-5551. |
| [21] | M. Amini, A. Tarassoli, S. Yousefi, et al., Suzuki-Miyaura cross-coupling reactions in water using in situ generated palladium(Ⅱ)-phosphazane complexes, Chin. Chem. Lett. 25 (2014) 166-168. |
| [22] | M. Jiang, H.J. Yang, Y. Li, Z.Y. Jia, H. Fu, Metal-free synthesis of substituted phenols from arylboronic acids in water at room temperature, Chin. Chem. Lett. 25 (2014) 715-719. |
| [23] | P. Anastas, N. Eghbali, Green chemistry: principles and practice, Chem. Soc. Rev. 39 (2010) 301-312. |
| [24] | X. Rao, C. Liu, Y. Zhang, Z. Gao, Z. Jin, Pd/C-catalyzed aerobic and ligand-free Suzuki reaction in water, Chin. J. Catal. 35 (2014) 357-361. |
| [25] | N. Liu, C. Liu, Z. Jin, Poly(ethylene glycol)-functionalized imidazolium salts- palladium-catalyzed Suzuki reaction in water, Green Chem. 14 (2012) 592-597. |
| [26] | C. Liu, Y.X. Zhang, N. Liu, J.S. Qiu, A simple and efficient approach for the palladium-catalyzed ligand-free Suzuki reaction in water, Green Chem. 14 (2012) 2999-3003. |