b Department of Microbiology, Kurukshetra University, Kurukshetra, India
The focus on the discovery and optimization of novel antimicrobial agents which are active against resistant strains is an active area of research [1]. The development of new synthetic methods leading to hybrid structures,which consist of different biologically active moieties in a single molecule,has attracted much attention. The heterocyclic pharmacophores are selected on the basis of their known bio profiles,so that the successive hybrid molecules may exhibit synergistic or additive pharmacological activities [2,3].
Multicomponent reactions (MCRs) approach has played an important role in modern drug discovery by introducing structural complexity and diversity and also offers various advantages like atom economy and high efficiency over conventional linear-type syntheses [4,5,6]. The role of click chemistry is also very significant in modern drug discovery. Biologically important compounds containing more than one pharmacophore have been developed by many researchers in a single step by combining MCRs with click reactions [7,8]. Deploying non-toxic,bio-degradable and biocompatible reaction media would be an added advantage for organic synthesis as it reduces environmental burden. Over the years,polyethylene glycol (PEG-400) and modified polyethylene glycol derivatives have become more popular alternate reaction media,due to their interesting properties like non-toxicity,biocompatibility,and bio-degradability when compared to other ‘neoteric solvents’ such as ionic liquids,super-critical fluids,and micellar systems [9]. Several reactions like substitution reactions [10],oxidation and reduction reactions [11],Heck reaction [12], asymmetric dihydroxylation [13],coupling reaction [14],Wacker reaction [15],and partial reductions of alkynes [16] were reported using PEG-400.
5-Arylidenethiazolidine-2,4-diones have been found to exhibit several pharmacological activities such as antidiabetic [17], antioxidant [18],cardiotonic [19],anti-inflammatory [20],anticancer [21] and also inhibits several targets such as HCV protease [22],β-lactamase [23],JNK-stimulating phosphatase-1 (JSP-1) [24],aldose reductase inhibitor [25],tyrosine phosphatases inhibitor [26],antimicrobial and antihyperglycemic agents [27]. In addition,5-arylidenethiazolidine-2,4-diones have substantial anti-proliferative effect on vascular smooth muscle [28] and also act as selective inhibitors of the PIM-1,PIM-2,and PIM-3 protein kinases [29]. 1,2,3-Triazole and its derivatives have also attracted the attention of organic and medicinal chemists due to their numerous biological activities such as anti-tubercular [30],anti-HIV [31],antifungal [32],antibacterial [33],anticancer activities [34],GABA [35] and glycosidase inhibitors [36]. The enhanced biological activities shown by 1,2,3-triazole ring are probably due to high dipole moment,hydrogen bonding capability, rigidity and stability underin vivo conditions [37].
1,2,3-Triazoles are extremely stable under basic and acid hydrolysis and also in reductive and oxidative conditions [38,39]. Moreover,1,2,3-triazoles are also incorporated as linker between two pharmacophores to give bifunctional drugs [40]. It was also found that N-CH2 bridge has been used for synthesizing complex molecules containing two active pharmacophores [41].
In view of the biological importance of 1,2,3-triazoles and 5-arylidenethiazolidine-2,4-diones,and in continuation of our interest in the development of medicinally important heterocycles [42],we decided to explore the synthesis of hybrid heterocycles comprising 1,2,3-triazoles and 5-arylidenethiazolidine-2,4-dione substructures. Such a strategy would provide access to a rapid synthesis of 1H-1,2,3-triazole tethered 5-arylidenethiazolidine-2,4-dione molecular conjugates which are otherwise accessible through multistep synthesis. 2. Experimental
Structures of all of the compounds were identified by their spectral data. Silica gel 60 F254(Precoated aluminium plates) from Merck were used to monitor reaction progress. Melting points were determined on Buchi Melting Point M-560 apparatus and are uncorrected. IR (KBr) spectra were recorded on Perkin Elmer FTIR spectrophotometer and the values are expressed asnmaxcm-1. The 1H NMR and 13C NMR spectra were recorded on Jeol JNM ECX-400P at 400 MHz and 100 MHz respectively. Mass spectra were recorded at Bruker Micro TOF Q - II. The chemical shift values are recorded ondscale and the coupling constants (J) are in Hertz. The aryl azides used in the synthesis of compounds (5a-5p) were prepared from aromatic amines by reported procedure [43].
General procedure for the synthesis of 5-(4-methylbenzylidene)-3-((1-(4-tolyl)-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-dione (5a): A mixture of thiazolidine-2,4-dione (1.0 equiv.),4-methylbenzaldehyde (1.0 equiv.),piperidine (1.0 equiv.) and 5 mL of PEG-400 was placed in a 50 mL round-bottomed flask and the contents were stirred magnetically in an oil-bath maintained at 120°C until 5-(4-methylbenzylidene)thiazolidine-2,4-dione (3) was formed (3-4 h),which was monitored by TLC using ethyl acetate-petroleum ether,(30:70,v/v) as eluent. Then,propargyl bromide (1.0 equiv.),4-methylphenyl azide (1.0 equiv.) (4) were added to the above reaction mixture followed by an aqueous solution of CuSO4·5H2O (10 mol%) and aqueous solution of sodium ascorbate (20 mol%). The reaction mixture was further heated at 120°C for 60 min. The progress of the reaction was monitored by TLC (ethyl acetate-petroleum ether,40:60,v/v). After completion of the reaction,the reaction mixture was allowed to cool at room temperature and was quenched with water (~5 mL). The precipitate formed was collected by filtration at pump and washed with water. The product so obtained,was purified by using flash column chromatography over silica gel (230-400 mesh) to afford pure product5a. Off-white solid; mp: 203°C; 1H NMR (400 MHz, CDCl3):dH8.00 (s,1H,Ar-H),7.87 (s,1H,C55C-H),7.56-7.54 (s,2H, Ar-H),7.37-7.35 (m,2H,Ar-H),7.27-7.23 (m,4H,Ar-H),5.10 (s, 2H,N-CH2 ),2.38 (s,3H,CH3),2.37 (s,3H,CH3); 13C NMR (100 MHz, CDCl3): d167.6,165.8,141.4,139.0,134.5,130.3,130.1,129.9, 121.5,120.5,119.8,36.4,21.5,21.0; IR (KBr,cm-1): nmax1745, 1684,1599,1336,1238,1136,1044. MS (ESI)m/z calcd. for C21H18N4O2S: 390.1150 HR-MS (ESI): 391.1225 [M++1].
By a similar procedure,5b-5pwere synthesized and characterized using 1H NMR,13C NMR,IR and mass. Spectral data for all other compounds and the procedure for antimicrobial screening were included in Supporting information [44,45,46,47,48]. 3. Result and discussion 3.1. Chemistry
The present manuscript reports the synthesis of targeted 5-(arylidene)-3-((1-aryl-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-diones,by a multicomponent domino process. Firstly,Knoevenagel condensation of thiazolidine-2,4-dione and aromatic aldehydes using piperidine as catalyst in PEG-400 at 120°C resulted in the formation of 5-(arylidene)thiazolidine-2,4-dione (3),which further reacts with propargyl bromide and 1-azido-4-methylbenzene (4a) in presence of CuSO4·5H2O and sodium ascorbate as catalysts in the same pot resulting in the formation of 5-(arylidene)-3-((1-aryl-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-diones (5) (Scheme 1).
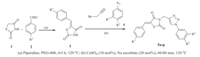
| Download: |
| Scheme 1.Synthesis of 5-(arylidene)-3-((1-aryl-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-diones (5a-5p)using domino process. | |
To search the optimal reaction conditions for the synthesis of 5, firstly Knoevenagel condensation of thiazolidine-2,4-dione (1.0 equiv.) and 4-methylbenzaldehyde (1.0 equiv.) was carried out in refluxing ethanol using piperidine (1.0 equiv.) as base [27]. The reaction was completed after 6 h as monitored by TLC (ethyl acetate: petroleum ether,40:60,v/v). Propargyl bromide (1.0 equiv.) and 1-azido-4-methylbenzene (1.0 equiv.) (4) were then added to the same pot followed by addition of aq. CuSO4·5H2O (10 mol%) and aq. sodium ascorbate (20 mol%). The reaction was not complete even after 12 h but yielded 50% of5aafter workup (Table 1,entry 1).
|
Table 1 Optimization table for the synthesis of5astarting using domino processa |
The formation of 5a was confirmed by 1H NMR and 13C NMR. A singlet at δ 8.00 for 1 proton in the 1H NMR spectra is assigned to proton on the C-5 carbon of triazole ring. Similarly a singlet at δ 7.87 for 1 proton is assigned to the exocyclic double bond. A singlet atd5.10 confirms the presence of N-CH2 group and two singlets at δ 2.37 and 2.38 are assigned to three protons each for two methyl groups attached to the phenyl ring. 13C NMR also shows characteristic peaks at δ 119.8 for C-5 of triazole ring,δ 36.4 for methylene carbon attached to nitrogen of thiazolidinedione ring and at δ 21.5 and 21.0 for carbons of two methyl groups,respectively. To improve the efficacy,the reaction was then attempted in other solvents like methanol,DMF,PEG-400 and PEG-600 (Table 1,entries 2-7). The highest yield of5a(90%) was obtained when the reaction was carried out in PEG-400 at 120°C (Table 1,entry 7). Thiazolidine-2,4-dione (1.0 equiv.),4-methylbenzaldehyde (1.0 equiv.) and piperidine (1.0 equiv.) as base were reacted in PEG-400 at 120°C. After completion of reaction as evident from TLC (ethyl acetate:petroleum ether,40:60,v/v) after 4 h,propargyl bromide (1.0 equiv.) and 1-azido-4-methylbenzene (1.0 equiv.) (4) were then added to the same pot followed by addition of aq. CuSO4·5H2O (10 mol%) and aq. sodium ascorbate (20 mol%). The reaction was completed after 60 min and results in the formation of 5a in 90% yield.
Subsequently,reactions of thiazolidine-2,4-dione and other aromatic aldehydes containing electron withdrawing and electron releasing substituents were carried out in PEG-400 using piperidine as base followed by addition of propargyl bromide,aryl azides, aq. CuSO4 and aq. sodium ascorbate in the same pot at 120°C. All the reactions were complete in less than 80 min and gave the desired products 5-(arylidene)-3-((1-aryl-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-diones (5a-5p) in high yields (CuSO4 ,Scheme 1).
|
CuSO4 Synthesis of 5-(arylidene)-3-((1-aryl-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-diones (5a-5p)using multicomponent domino reaction. |
All the novel compounds were characterized by 1H NMR,13C NMR,IR and ESI-MS/HRMS. The formation of triazole ring was confirmed by the resonance of H-5 of the triazole ring in the aromatic region in the 1H NMR spectra. The structures were further supported by the 13C NMR spectra,which confirmed the C-skeleton of triazole derivatives 5a-5p . ESI-MS/HRMS of all compounds showed [M++Na] and [M++1]. The structure was finally established by single crystal X-ray diffraction study of 5m which confirmed the regioselectivity of the product (Fig. 1).
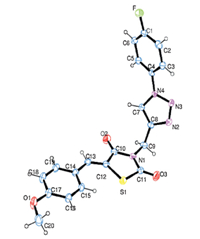
| Download: |
| Fig. 1. Crystal structure of compound5mby single crystal X-ray diffraction study. | |
A total of 16 chemical compounds were screened for their antibacterial and antifungal activity. Tested chemical compounds possessed variable antibacterial activity against Gram-positive (Staphylococcus aureus,Bacillus subtilis) bacteria. However,they did not exhibit any activity against Gram-negative (Escherichia coliand Pseudomonas aeruginosa) bacteria. Positive controls produced significantly sized inhibition zones against the tested bacteria and fungi. However,negative controls did not show observable inhibitory effect against any of the test organisms as shown in Table 3.
|
Table 3 Antibacterial activity of compounds 5a-5p. |
Compounds 5a-5p showed zone of inhibition ranging between 13.3 mm and 22.3 mm against the Gram-positive bacteria. On the basis of zone of inhibition produced against the test bacterium,five compounds namely,5g,5h,5i,5k and 5o were found to be most active against S. aureus and B. subtilis with zone of inhibition ranging between 18.3 mm and 22.3 mm,respectively. Compound 5h shows more than 80% inhibition of bacterial growth. However other tested compounds showed moderate antibacterial activity (Table 3). Minimum inhibitory concentration (MIC) of all compounds 5a-5p was measured against Gram-positive bacteria, as shown in Table 3. The MIC of chemical compounds ranged between 32 and 256mg/mL against Gram-positive bacteria. Four compounds 5f,5g,5i,5k were found to be best as they exhibited the MIC of 64mg/mL againstS. aureusandB. subtilis whereas5h was found to be best againstS. aureusandB. subtilis with lowest MIC of 32mg/mL (Table 3). Presence of electron withdrawing substituent on the phenyl ring attached to the exocyclic double bond enhances the activity of compounds. The enhanced activity of 5h is due to substitution of three halogen substituents on both the phenyl rings. 3.2.2. Antifungal activity
All the 16 compounds 5a-5p were also tested for theirin vitro antifungal activity against two fungal strains,namely,Aspergillus nigerandAspergillus flavusspecies through poisoned food method. Standard drug fluconazole was used for comparison of antifungal activity shown by the compounds and results were recorded as percentage (%) of mycelial growth inhibition. It can be inferred from Table 4 that all the compounds 5a-5p showed variable antifungal activity against two pathogens. It can be inferred by comparison of the results that seven compounds namely 5d,5f,5i, 5m,5n,5o,and 5p showed high antifungal activity with>50% inhibition of mycelial growth against the two fungi in comparison with the standard drug. Other compounds also showed good antifungal activities as summarized in Table 4.
|
Table 4 Antifungal activity of compounds 5a-5p through poisoned food method. |
The present study offers an efficient and convenient multicomponent domino process for the synthesis of (Z)-5-(arylidene)-3-((1-aryl-1H-1,2,3-triazol-4-yl)methyl)thiazolidine-2,4-diones (5a-5p).Among all the tested chemical compounds,compound 5h showed good antibacterial and 5o showed good antifungal activity. The hybrid molecule shows good antimicrobial activities as compared to their thiazolidinedione substructure used for the synthesis of new antimicrobials. Hence this study has widened the scope of developing thiadiazolidine-2-4-dione based derivatives as promising antibacterial agents.
Acknowledgment
JS and HS thank UGC,New Delhi,India for the grant of Junior and Senior Research Fellowships.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet. 2014.09.006.
| [1] | F.L. Gouveia, R.M.B. De Oliveira, T.B. De Oliveira, et al., Synthesis, antimicrobial and cytotoxic activities of some 5-arylidene-4-thioxo-thiazolidine-2-ones, Eur. J. Med. Chem. 44 (2009) 2038-2043. |
| [2] | E.M. Guantai, K. Ncokazi, T.J. Egan, et al., Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds, Bioorg. Med. Chem. 18 (2010) 8243-8256. |
| [3] | C.A. Fraga, Drug hybridization strategies: before or after lead identification? Expert Opin. Drug Discov. 4 (2009) 605-609. |
| [4] | J.E. Biggs-Houck, A. Younai, J.T. Shaw, Recent advances in multicomponent reactions for diversity-oriented synthesis, Curr. Opin. Chem. Biol. 14 (2010) 371-382. |
| [5] | L.A. Marcaurelle, M.A. Foley, The evolving role of molecular diversity in drug discovery, Curr. Opin. Chem. Biol. 14 (2010) 285-288. |
| [6] | N. Dahan-Farkas, C. Langley, A.L. Rousseau, et al., 6-Substituted imidazo[1,2- a]pyridines: synthesis and biological activity against colon cancer cell lines HT-29 and Caco-2, Eur. J. Med. Chem. 46 (2011) 4573-4583. |
| [7] | (a) D.J. Ramón, M. Yus, Asymmetric multicomponent reactions (AMCRs): the new frontier, Angew. Chem. Int. Ed. Engl. 44 (2005) 1602-1634; |
| [8] | (a) J. Sindhu, H. Singh, J.M. Khurana, C. Sharma, K.R. Aneja, Multicomponent synthesis of novel 2-aryl-5-((1-aryl-1H-1,2,3-triazol-4-yl)methylthio)-1,3,4-oxadiazoles using CuI as catalyst and their antimicrobial evaluation, Aust. J. Chem. 66 (2013) 710-717; |
| [9] | J. Chen, S.K. Spear, J.G. Huddleston, R.D. Rogers, Polyethylene glycol and solutions of polyethylene glycol as green reaction media, Green Chem. 7 (2005) 64-82. |
| [10] | P. Ferravoschi, A. Fiecchi, P. Grisenti, E. Santaniello, S. Trave, Polyethylene glycols as solvents for anionic activation: synthesis of thioacetates by means of potassium thioacetate in polyethylene glycol 400, Synth. Commun. 17 (1987) 1569- 1575. |
| [11] | J.R. Blanton, The selective reduction of aldehydes using polyethylene glycol- sodium borohydride derivatives as phase transfer reagents, Synth. Commun. 27 (1997) 2093-2102. |
| [12] | S. Chandrasekhar, Ch. Narsihmulu, S.S. Sultana, N.R.K. Reddy, Poly(ethylene glycol) (PEG) as a reusable solvent medium for organic synthesis, application in the Heck reaction, Org. Lett. 4 (2002) 4399-4401. |
| [13] | S. Chandrasekhar, Ch. Narsihmulu, S.S. Sultana, N.R.K. Reddy, Osmium tetroxide in poly(ethylene glycol) (PEG): a recyclable reaction medium for rapid asymmetric dihydroxylation under sharpless conditions, Chem. Commun. (2003) 1716- 1717. |
| [14] | (a) V.V. Namboodiri, R.S. Varma, Microwave-accelerated Suzuki cross-coupling reaction in polyethylene glycol (PEG), Green Chem. 3 (2001) 146-148; |
| [15] | A. Haimov, R. Neumann, Polyethylene glycol as a non-ionic liquid solvent for polyoxometalate catalyzed aerobic oxidation, Chem. Commun. (2002) 876-877. |
| [16] | S. Chandrasekhar, Ch. Narsihmulu, G. Chandrasekhar, T. Shyamsundar, Pd/CaCO3 in liquid poly(ethylene glycol) (PEG): an easy and efficient recycle system for partial reduction of alkynes to cis-olefins under a hydrogen atmosphere, Tetrahedron Lett. 45 (2004) 2421-2423. |
| [17] | S.R. Pattana, P. Kekareb, A. Patilc, A. Nikaljec, B.S. Kitturd, Studies on the synthesis of novel 2,4-thiazolidinedione derivatives with antidiabetic activity, Iran. J. Pharm. Sci. 5 (2009) 225-230. |
| [18] | R. Ottana`, R. Maccari, M. Giglio, et al., Identification of 5-arylidene-4-thiazolidinone derivatives endowed with dual activity as aldose reductase inhibitors and antioxidant agents for the treatment of diabetic complications, Eur. J. Med. Chem. 46 (2011) 2797-2806. |
| [19] | A. Andreani, M. Rambaldi, A. Locatelli, et al., Synthesis of lactams with potential cardiotonic activity, Eur. J. Med. Chem. 28 (1993) 825-829. |
| [20] | C.D. Barros, A.A. Amato, T.B. Oliveira, et al., Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARgamma ligands, Bioorg. Med. Chem. 18 (2010) 3805-3811. |
| [21] | Z. Beharry, M. Zemskova, S. Mahajan, et al., Novel benzylidene-thiazolidine-2,4- diones inhibit Pim protein kinase activity and induce cell cycle arrest in leukemia and prostate cancer cells, Mol. Cancer Ther. 8 (2009) 1473-1483. |
| [22] | W.T. Sing, C.L. Lee, S.L. Yeo, S.P. Lim, M.M. Sim, Arylalkylidene rhodanine with bulky and hydrophobic functional group as selective HCV NS3 protease inhibitor, Bioorg. Med. Chem. Lett. 11 (2001) 91-94. |
| [23] | E.B. Grant, D. Guiadeen, E.Z. Baum, et al., The synthesis and SAR of rhodanines as novel class C beta-lactamase inhibitors, Bioorg. Med. Chem. Lett. 10 (2000) 2179-2182. |
| [24] | N.S. Cutshall, C. O'Day, M. Prezhdo, Rhodanine derivatives as inhibitors of JSP-1, Bioorg. Med. Chem. Lett. 15 (2005) 3374-3379. |
| [25] | S.V. Sambasivarao, L.K. Soni, A.K. Gupta, P. Hanumantharao, Quantitative structure- activity analysis of 5-arylidene-2,4-thiazolidinediones as aldose reductase inhibitors, Bioorg. Med. Chem. Lett. 16 (2006) 512-520. |
| [26] | R. Maccari, P. Paoli, R. Ottana, et al., 5-Arylidene-2,4-thiazolidinediones as inhibitors of protein tyrosine phosphatases, Bioorg. Med. Chem. 15 (2007) 5137-5149. |
| [27] | V.R. Avupati, R.P. Yejella, A. Akula, et al., Synthesis, characterization and biological evaluation of some novel 2,4-thiazolidinediones as potential cytotoxic, antimicrobial and antihyperglycemic agents, Bioorg. Med. Chem. Lett. 22 (2012) 6442- 6450. |
| [28] | J.D. Peuler, S.M. Phare, A.R. Lannussi, M.J. Hoderek, Differential inhibitory effects of antidiabetic drugs on arterial smooth muscle cell proliferation, Am. J. Hypertens. 9 (1996) 188-192. |
| [29] | L.A. Dakin, M.H. Block, H. Chen, et al., Discovery of novel benzylidene-1,3- thiazolidine-2,4-diones as potent and selective inhibitors of the PIM-1, PIM-2, and PIM-3 protein kinases, Bioorg. Med. Chem. Lett. 22 (2012) 4599-4604. |
| [30] | C. Gill, G. Jadhav, M. Shaikh, et al., Clubbed [1-3] triazoles by fluorine benzimidazole: a novel approach to H37Rv inhibitors as a potential treatment for tuberculosis, Bioorg. Med. Chem. Lett. 18 (2008) 6244-6247. |
| [31] | F. de, C. da Silva, M.C.B.V. de Souza, I.I.P. Frugulhetti, et al., Synthesis, HIV-RT inhibitory activity and SAR of 1-benzyl-1H-1, 2,3-triazole derivatives of carbohydrates, Eur. J. Med. Chem. 44 (2009) 373-383. |
| [32] | N.G. Aher, V.S. Pore, N.N. Mishra, et al., Synthesis and antifungal activity of 1,2,3- triazole containing fluconazole analogues, Bioorg. Med. Chem. Lett. 19 (2009) 759-763. |
| [33] | B.S. Holla, M. Mahalinga, M.S. Karthikeyan, et al., Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles, Eur. J. Med. Chem. 40 (2005) 1173-1178. |
| [34] | M.S. Alam, J. Huang, F. Ozoe, F. Matsumura, Y. Ozoe, Synthesis, 3D-QSAR, and docking studies of 1-phenyl-1H-1,2,3-triazoles as selective antagonists for beta3 over alpha1beta2gamma2 GABA receptors, Bioorg. Med. Chem. 15 (2007) 5090- 5104. |
| [35] | R. Périon, V. Ferrières, M.I. García-Moreno, et al., 1,2,3-Triazoles and related glycoconjugates as new glycosidase inhibitors, Tetrahedron 61 (2005) 9118- 9128. |
| [36] | A. Kamal, N. Shankaraiah, V. Devaiah, et al., Synthesis of 1,2,3-triazole-linked pyrrolobenzodiazepine conjugates employing ‘click' chemistry: DNA-binding affinity and anticancer activity, Bioorg. Med. Chem. Lett. 18 (2008) 1468-1473. |
| [37] | H.C. Kolb, K.B. Sharpless, The growing impact of click chemistry on drug discovery, Drug Discov. Today 8 (2003) 1128-1137. |
| [38] | N.S. Vatmurge, B.G. Hazra, V.S. Pore, et al., Deshpande, synthesis and antimicrobial activity of beta-lactam-bile acid conjugates linked via triazole, Bioorg. Med. Chem. Lett. 18 (2008) 2043-2047. |
| [39] | M. Whiting, J. Muldoon, Y.C. Lin, et al., Inhibitors of HIV-1 protease by using in situ click chemistry, Angew. Chem. Int. Ed. 45 (2006) 1435-1439. |
| [40] | (a) J. Zhang, H. Zhang, W.X. Cai, et al., ‘Click' D(1) receptor agonists with a 5- HT(1A) receptor pharmacophore producing D(2) receptor activity, Bioorg. Med. Chem. 17 (2009) 4873-4880; (b) R. Jagasia, J.M. Holub, M. Bollinger, K. Kirshenbaum, M.G. Finn, Peptide cyclization and cyclodimerization by Cu(I)-mediated azide-alkyne cycloaddition, J. Org. Chem. 74 (2009) 2964-2974. |
| [41] | K. Kumar, S. Sagar, L. Esau, M. Kaur, V. Kumar, Synthesis of novel 1H-1,2,3-triazole tethered C-5 substituted uracil-isatin conjugates and their cytotoxic evaluation, Eur. J. Med. Chem. 58 (2012) 153-159. |
| [42] | (a) H. Singh, J. Sindhu, J.M. Khurana, Efficient, green and regioselective synthesis of 1,4,5-trisubstituted-1,2,3-triazoles in ionic liquid [bmim]BF4 and in taskspecific basic ionic liquid [bmim]OH, J. Iran. Chem. Soc. 10 (2013) 883-888; |
| [43] | N.D. Obushak, N.T. Pokhodylo, N.I. Pidlypnyi, V.S. Matiichuk, Synthesis of 1,2,4- and 1,3,4-oxadiazoles from 1-aryl-5-methyl-1H-1,2,3-triazole-4-carbonyl chlorides, Russ. J. Org. Chem. 44 (2008) 1522-1527. |
| [44] | K.R. Aneja, C. Sharma, R. Joshi, Fungal infection of the ear: a common problem in the North Eastern part of Haryana, Int. J. Pediatr. Otorhinolaryngol. 74 (2010) 604-607. |
| [45] | I. Ahmad, A.Z. Beg, Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens, J. Ethnopharmacol. 74 (2001) 113-123. |
| [46] | J.M. Andrews, Determination of minimum inhibitory concentrations, J. Antimicrob. Chemother. 48 (2001) 5-16. |
| [47] | National Committee for Clinical Laboratory Standards, Method for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically; Approved Standards, Villanova, PA, fifth ed., 2000. |
| [48] | S.K.S. Al-Burtamani, M.O. Fatope, R.G. Marwah, A.K. Onifade, S.H. Al-Saidi, Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman, J. Ethnopharmocol. 96 (2005) 107-112. |








