b Shenzhen Haibin Pharmaceutical Co., Ltd., Shenzhen 518081, China
Conventional direct energy conversion methods are mainly utilizing thermoelectric materials and their chemical structures, which have the capability of scavenging electrical energy based on the Seebeck effect [1, 2]. The emergence of nanostructured materials,as building blocks for clean energy-harvesting assemblies, leads to new ways for the development of renewable energy resources. Recently,Chen et al. [3] investigated a novel energy harvesting mechanism based on the capacitive effect of a liquid- solid interface in a nano-confinement. Jiang et al. successfully converted Gibbs free energy from an ion-concentration gradient into electricity in a polymer nanofluidic system. The maximum production capability with the individual polymer nanopore approach was ~ 350 pW[4]. The nanopores offer nano-confinement that plays an important role in energy generation with synthetic nanopores emerging as promising materials to mimic such asymmetric transport phenomena observed in biological ion channels [5]. Such ion current rectification behavior was mainly observed in synthetic conical nanoporeswith the overlapping of the electrical double-layers (EDLs) and asymmetric surface charge in nanoscale geometries,which leads to electrostatic interaction between the ions traversing the conical nanopore and the surface charge at the tip opening [6, 7].
Previous studies endeavored to tune the ionic current rectification properties and the ion transport selectivity by chemical modification of the nanopore surface with various functional groups [8]. While in the presence of a concentration gradient,the ions diffused spontaneously across the channel [9- 12]. Moreover,transpore-rectifying nanopores and nanochannels find application in sensing [13, 14, 15],nanofluidic circuits [16, 17], and energy conversion [4]. In our previous work,chemically modified,glass conical nanopore channels were used to mimic the biological ion channels [18, 19]. The preparation of glass conical nanopores is facile and inexpensive and the formed channels are compact and mechanically robust. In addition,the hydroxylterminated silica surface of a glass conical nanopore channel is easily coated with silane self-assembled monolayers and further functionalized by covalent modification [20]. In this paper,we present an experimental study of the influence of concentration gradients on the ion current rectification and the ion transport selectivity of charged single glass conical nanopore channels. The degree of ICR is enhanced with the increasing forward concentration difference. An unusual rectification inversion is observed when the concentration gradient is reversely applied. The maximum energy production capability of the glass conical nanopore was 104 pW. The present study advances our understanding of the ICR in asymmetric nanofluidic channels associated with the ion concentration difference and provides insight into the rectifying biological ion channels.
2. Experimental 2.1. Preparation of single glass conical nanopore channels and estimation of the orifice radiusThe single glass conical nanopore channels were prepared from glass capillaries according to the method reported by White and coworkers with slight modifications [21]. A platinum wire (diameter 25 mm,Alfa Aesar) was first electrochemically etched in 15% CaCl2 to obtain a sharpened tip,and then sealed into a glass capillary (1.35-mm outer diameter,0.95-mm inner diameter, Hirschmann,Germany). After that,the Pt sealed in glass was pulled out and etched to obtain single conical glass nanopore channel [22, 23]. The radius of the pore is determined by measuring the steady-state diffusion-limited current of the Pt disk electrode prior to etching according to the following equation [24]

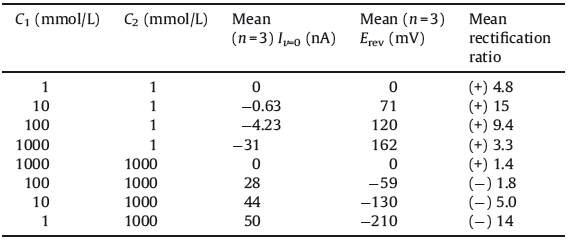
Glass nanopore channels were filled with electrolyte using a 100 μL microsyringe. After removal of any remaining air bubbles inside the channels by a brief and mild sonication,an Ag/AgCl electrode (0.5 mm diameter) was inserted and served as the working electrode,and another Ag/AgCl electrode (0.5 mm diameter) was placed in the bulk solution as an auxiliary/reference electrode. In all cases,the nanopore channels were filled with the same electrolyte as the bulk solution. Linear sweep voltammetry experiments were carried out with a CHI 660C electrochemical workstation (Shanghai CHI Instrument Co.,Ltd.,China). The measurement of the resulting ion current flowing through the nanopore channel was performed by scanning the voltage from +1 V to +1 V with a scanning rate 100 mV/s. The 1 mol/L KCl/ 10 mmol/L phosphate buffer (pH 7.4) solution was used as the stock solution to prepare other KCl solutions with different concentrations by stepwise dilution. The concentrations of the electrolyte in contact with the tip and base were defined as C1 and C2,respectively (Scheme 1).
|
Download:
|
| Scheme 1. Set up of the experiment. | |
The ion current rectificationwas observed since the surface of the glass nanopore channel was negatively charged due to the dissociation of silica hydroxyl groups (Si-OH) in the electrolyte. The rectification ratio is defined as a ratio (I (‘‘on’’ state)/I (‘‘off’’ state)) of the absolute value of current recorded for voltage of the same value,but opposite polarity (±1 V). When the KCl concentration was low,the I-V curve was non-linear,and the current recorded at negative voltage was much higher than that at positive voltage with the rectification ratio of ~4.8 (Fig. 1A). When the electrolyte concentration was high,the pore rectified less,and the rectification ratio decreased as the increase of the KCl concentration (Fig. 1B). The electric double-layer thickness was significantly reduced with the increase of electrolyte concentration. The more cross-sectional area was occupied by the double-layer thickness,the greater the effect on the ion transport through the conical nanopore became. The result shown here was in good agreement with that reported by Wei et al.[25].

|
Download:
|
| Fig. 1. Current rectification of glass nanopore channels with tip radius 16 nm. (A) I-V curve of a single glass conical nanopore channel under symmetric electrolyte conditions 1 mmol/L KCl,pH 7.4. (B) Current rectification ratios at various KCl concentrations (n = 3). | |
The rectification properties under the concentration gradient between two sides of the nanopore channel were investigated. There are two ways to apply the salt concentration gradient: with C1 < C2 or with C1 > C2. When C1 > C2,the nanopore channel shows high conductivity (‘‘on’’ state) at V < 0 and lower conductivity at V > 0 (‘‘off’’ state),(Fig. 2A). While C1 < C2,the current rectification shows reversed direction with much higher ion currents recorded at the positive potential than at the negative potential (Fig. 2B).

|
Download:
|
| Fig. 2. I-V curves of a single glass conical nanopore channel (orifice radius 16 nm) recorded at asymmetric electrolyte concentrations. (A) I-V curves recorded with C1 > C2, C1 = 10,100,1000 mmol/L,C2 = 1 mmol/L; (B) I-V curves recorded with C1 < C2,C1 = 100,10,1 mmol/L,C2 = 1 mol/L; (C) I-V curves recorded with C1 = 1 mmol/L,C2 = 1 mol/L (blue dot line),C1 = 1 mol/L,C2 = 1 mmol/L (red line). | |
Therefore,the ion current rectification depends on the direction of the concentration gradient. Under the asymmetric electrolyte solution with C1 = 1000 mmol/L,C2 = 1 mmol/L (Fig. 2C,red line), the current rectification ratio was ~3.3. When C1 = 1 mmol/L, C2 = 1000 mmol/L (Fig. 2C,blue dot line),it was found that the rectification direction was reversed with rectification ratio ~14 several times higher than that of the symmetric concentration C1 = C2 = 1 mmol/L (with current rectification ratio of ~4.8).
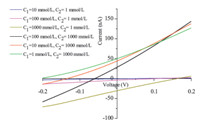
Furthermore,I-V curves were measured under a series of concentration gradients at 0.2 V transmembrane potential (Fig. 3) and detailed data are showed in Table 1. When the concentration is different on both sides of the nanopore,even without applying any voltage,the current value was not zero (this potential is defined as ‘‘zero-volt current’’ I0). In the case of the same salt concentrations on both sides of the nanopore channel,the zero-volt current was zero since there was no concentration gradient and thus no driving force for ion transport through the nanopore channels (Fig. 1A).
|
Download:
|
| Fig. 3. I-V curves recorded from -0.2 V to +0.2 V at different concentration gradients. | |
| Table 1 Values of zero-volt currents,reversal potentials and rectification ratios under different concentration gradients of a single glass conical nanopore channel with excess negative surface charge. |
A potential was observed at which the current was zero (this current is defined as ‘‘zero-current voltage’’ (reversal potential, Erev)). The concentration gradient between two sides of the nanopore channel could tune the rectification degree and even the rectification direction if the concentration gradient was great enough. The greater the concentration gradient was,the larger the absolute values of the zero-volt current and reversal potentials were,and the higher the selectivity of the pore was. The glass conical nanopore fluidic systems with electrolyte concentration gradient hold the promise for use as a nanofluidic power source. The maximum energy production capability of the nanopore fluidic system can be calculated by the equation,Pmax = I0 × Erev [4]. Compared to polymer nanopores,it seems that the glass conical nanopore possess a much higher Pmax. When we applied asymmetric electrolyte concentrations (C1 = 1 mmol/L, C2 = 1000 mmol/L) on both sides of the nanopore,the maximum energy production capability of the nanopore fluidic system was the highest,about 104 pW.
4. ConclusionWe have demonstrated experimentally that the concentration gradient could pump energy into the system without the need of any external forces via a single glass conical nanopore,and the magnitude of diffusion flow depends on the strength of the concentration gradient. Ion selectivity depends on the direction of the applied salt gradient,and is a consequence of geometric effects and the electrostatic interactions between the nanopore fixed charges and the salt ions. Furthermore,the glass conical nanopore can generate much higher energy output than present polymer nanopores. The study presented here will benefit the design of nanofluidic devices for nanoscale chemical delivery.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21375111,21127005, 20975084),the Ph.D. Programs Foundation of the Ministry of Education of China (No. 20110121110011). We thank Professor Richard N. Zare for the illumination in applications of nanopores, Professor Henry S. White and his coworkers for sharing the experience of glass nanopore fabrication. We are also grateful to Dr. Jia-Hai Wang and Dr. Yu-Luan Chen for helpful discussions.
| [1] | B. Kumar, S.W. Kim, Energy harvesting based on semiconducting piezoelectric ZnO nanostructures, Nano Energy 1 (2012) 342-355. |
| [2] | C. Xu, C.F. Pan, Y. Liu, Z.L. Wang, Hybrid cells for simultaneously harvesting multi-type energies for self-powered micro/nanosystems, Nano Energy 1 (2012) 259-272. |
| [3] | B.X. Xu, L. Liu, H. Lim, Y. Qiao, X. Chen, Harvesting energy from low-grade heat based on nanofluids, Nano Energy 1 (2012) 805-811. |
| [4] | W. Guo, L.X. Cao, J.C. Xia, et al., Energy harvesting with single-ion-selective nanopores: a concentration-gradient-driven nanofluidic power source, Adv. Funct. Mater. 20 (2010) 1339-1344. |
| [5] | Z.S. Siwy, Ion-current rectification in nanopores and nanotubes with broken symmetry, Adv. Funct. Mater. 6 (2006) 735-746. |
| [6] | Z. Siwy, E. Heins, C.C. Harrell, P. Kohli, C.R. Martin, Conical-nanotube ion-current rectifiers: the role of surface charge, J. Am. Chem. Soc. 35 (2004) 10850-10851. |
| [7] | Z.S. Siwy, C.R. Martin, Tuning ion current rectification in synthetic nanotubes, Controlled Nanoscale Motion, vol. 711, Springer, Berlin, Heidelberg, 2007, pp. 349-365. |
| [8] | M. Ali, B. Schiedt, K. Healy, R. Neumann,W. Ensinger, Modifying the surface charge of single track-etched conical nanopores in polyimide, Nanotechnology 8 (2008) 085713. |
| [9] | Z. Siwy, I.D. Kosińska, A. Fuliński, C.R. Martin, Asymmetric diffusion through synthetic nanopores, Phys. Rev. Lett. 4 (2005), 048102/1-048102/4. |
| [10] | R.Y. Chein, B.G. Chung, Numerical study of ionic current rectification through nonuniformly charged micro/nanochannel systems, J. Appl. Electrochem. 43 (2013) 1197-1206. |
| [11] | W. Guo, Y. Tian, L. Jiang, Asymmetric ion transport through ion-channel-mimetic solid-state nanopores, Acc. Chem. Res. 46 (2013) 2834-2846. |
| [12] | I.D. Kosinska, A. Fulinski, Asymmetric nanodiffusion, Phys. Rev. E: Stat. Nonlin. Soft Matter Phys. 72 (1) (2005), 011201/1-011201/7. |
| [13] | G.X. Li, X.Q. Lin, A glass nanopore electrode for single molecule detection, Chin. Chem. Lett. 21 (2010) 1115-1118. |
| [14] | B. Vilozny, A.L. Wollenberg, P. Acis, et al., Carbohydrate-actuated nanofluidic diode: switchable current rectification in a nanopipette, Nanoscale 5 (2013) 9214-9221. |
| [15] | H.C. Zhang, X. Hou, L. Zeng, et al., Bio-inspired artificial single ion pump, J. Am. Chem. Soc. 43 (2013) 16102-16110. |
| [16] | M. Ali, S. Mafe, P. Ramirez, R. Neumann, W. Ensinger, Logic gates using nanofluidic diodes based on conical nanopores functionalized with polyprotic acid chains, Langmuir 25 (2009) 11993-11997. |
| [17] | J. Cervera, P. Ramirez, S. Mafe, P. Stroeve, Asymmetric nanopore rectification for ion pumping, electrical power generation, and information processing applications, Electrochim. Acta 56 (2011) 4504-4511. |
| [18] | L.X. Zhang, X.H. Cao, Y.B. Zheng, Y.Q. Li, Covalent modification of single glass conical nanopore channel with 6-carboxymethyl-chitosan for pH modulated ion current rectification, Electrochem. Commun. (2010) 1249-1252. |
| [19] | L.X. Zhang, S.L. Cai, Y.B. Zheng, X.H. Cao, Y.Q. Li, Smart homopolymer poly (2- (dimethylamino) ethyl methacrylate) modification to single glass conical nanopore channels: proton and thermo dual-stimuli actuated highly efficient iongating, Adv. Funct. Mater. 11 (2011) 2103-2107. |
| [20] | Y.Q. Li, Y.B. Zheng, R.N. Zare, Electrical, optical, and docking properties of conical nanopores, ACS Nano 6 (2012) 993-997. |
| [21] | B. Zhang, J. Galusha, P.G. Shiozawa, et al., Bench-top method for fabricating glasssealed nanodisk electrodes, glass nanopore electrodes, and glass nanopore membranes of controlled size, Anal. Chem. 13 (2007) 4778-4787. |
| [22] | X.H. Cao, L.X. Zhang, W.P. Cai, Y.Q. Li, Amperometric sensing of dopamine using a single-walled carbon nanotube covalently attached to a conical glass micropore electrode, Electrochem. Commun. 12 (2010) 540-543. |
| [23] | L.X. Zhang, X.H. Cao, W.P. Cai, Y.Q. Li, Observations of the effect of confined space on fluorescence and diffusion properties of molecules in single conical nanopore channels, J. Fluoresc. 5 (2011) 1865-1870. |
| [24] | B. Zhang, Y.H. Zhang, H.S. White, Steady-state voltammetric response of the nanopore electrode, Anal. Chem. 2 (2006) 477-483. |
| [25] | C. Wei, A.J. Bard, S.W. Feldberg, Current rectification at quartz nanopipette electrodes, Anal. Chem. 22 (1997) 4627-4633. |