b Shimadzu Global COE for Application & Technical Development, Shimadzu (China) Co., Ltd., Shanghai 200052, China
Interest in food safety is rapidly increasing all over the world. Chloramphenicol is one of the mostly used antibiotics. Honey is a common food product in the world and large amount of honey is exported from,and imported by many countries. There are regulations for chloramphenicol in honey in EU [1],etc. Generally, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is used for analysis of chloramphenicol in honey after off-line solid phase extraction (SPE) as a pretreatment method [2, 3, 4]. Pretreatment of food samples is one of the key issues for dealing with trace amount of analytes. Development of new methods for faster analysis of contaminants in food samples has been greatly expected. In our laboratory,we have developed on-line pretreatment HPLC systems for various analytes [5, 6, 7, 8, 9]. On-line pretreatment with a pretreatment column is a time-saving method compared with generally used pretreatment methods such as liquid-liquid extraction,off-line solid-phase extraction. Previously, Yamamoto et al. developed methylcellulose-immobilized restricted-access material (MC-ODS) and evaluated the column for HPLC analysis of various drugs in plasma [10]. Kawano et al. used the MC-ODS column for LC-MS determination of drugs spiked in plasma and the method was of good precision,accuracy,and reproducibility [11]. Based on our previous works [11, 12],we evaluated the on-line pretreatment LC-MS/MS system with the MC-ODS column to remove sugars in honey for minimizing sample pretreatment in this study.
2. ExperimentalChloramphenicol and chloramphenicol-d5 were purchased from Sigma-Aldrich (St. Louis,MO,USA). Water for dilution of honey samples and mobile phases was prepared with Merck Millipore (Darmstadt,Germany) Milli-Q system. Acetonitrile (ACN,LC-MS grade) and methanol were purchased from Merck Millipore.
Honey samples were purchased at the local supermarkets. Standard chloramphenicol solution was prepared by dissolving and diluting with methanol. Honey sample (1 g),chloramphenicol (≥200 pg) and chloramphenicol-d5 (10 ng) were dissolved in Milli- Q water (10 mL).
The liquid chromatograph was a Shimadzu (Kyoto,Japan) LC- 30A system with a CBM-20A communications bus module,two LC-30AD pumps for gradient elution,an LC-20AB pump for pretreatment,an SIL-30AC autosampler,a CTO-30A column oven,and a 6-port flow changeover valve FCV-32AH. A Shim-pack MAYI-ODS (10 mm × 2.0 mm,particle size: 50 mm,pore size: 12 nm) was held at room temperature (ca. 30 ℃) with an eluent composition of water/ACN (95/5) at a flow rate of 1 mL/min. The injection volume was 20 mL. After extraction (1 min) the changeover valve was switched and mobile phase for analysis was introduced at a flow-rate of 0.4 mL/min. The analytical column was the Shim-pack XR-ODS (75 mm × 2.0 mm,particle size: 2.2 μm) and the column temperature was 40 ℃. Mobile phase A was water. Mobile phase B was ACN. Following gradient program was used: 5% ACN (0-1 min)-95% ACN (2.5-3 min)-5% ACN (3.01-5 min). The triple quadrupole mass spectrometer was LCMS-8080 equipped with an electrospray ionization (ESI) interface. Ionization voltage was -3.5 kV (negative mode). Neblizing gas flow,heating gas flow and curtain gas flow were 2 L/min,12 L/min and 3 L/min, respectively. Probe temperature and HSID temperature were 400 ℃ and 200 ℃,respectively. MRM transitions for chloramphenicol and chloramphenicol-d5 were m/zm/z 321.20 > 152.25 (m/z 321.20 > 257.15 for confirmation),and m/z 326.1 > 157.1, respectively. Dwell time was 100 ms for all transitions.
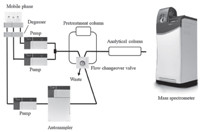
3. Results and discussionThe on-line pretreatment system was based on a columnswitching HPLC as shown in Fig. 1 (on-line pretreatment LC-MS/ MS system). After injection of samples,chloramphenicol is trapped on the ODS surface of the MC-ODS column,while sugars are removed without retention on the pretreatment column because there is no hydrophobic interaction. Then the 6-port flow changeover valve is switched and chloramphenicol is eluted from the MC-ODS column and introduced to the analytical column and the mass spectrometer.
|
Download:
|
| Fig. 1. On-line pretreatment LC-MS/MS system. | |
RepresentativeMRMchromatograms (m/z 321.20 > 152.25) of honey samples spiked with chloramphenicol are shown in Fig. 2 (MRM chromatograms of chloramphenicol ((a) 20 pg/mL,spiked, (b) 2000 pg/mL,spiked)). Retention time of chloramphenicol was 2.65 min. Another peak at 2.85 min was observed with honey samples. Amatrix-matched calibration curve of chloramphenicol in honey was constructed ranging from 20 pg/mL to 5000 pg/mL by internal standard method with chloramphenicol-d5. Correlation coefficient was 0.9994. Intra-day precision and accuracy (n = 5 at each concentration level) are shown in Table 1 (intra-day precision and accuracy). Excellent precision and accuracy were maintained. The lower limit of quantitation (LLOQ) of chloramphenicol was 20 pg/mL (=0.2 μg/kg honey). According to the EU regulation,the MRPL (minimum required performance limits) of chloramphenicol in honey is 0.3 μg/kg [1]. By comparison of peak area at 1000 pg/mL between standard chloramphenicol solution and honey sample spiked with chloramphenicol,recovery of chloramphenicolwas 78%. Inter-day precision and accuracy (3 days,n = 15 at each concentration level) are summarized in Table 2 (inter-day precision and accuracy). Values for precision were better than 11% and those for accuracy were between 92 and 111%. Eight different real honey samples were diluted with water and analyzed by on-line pretreatment LC-MS/MS. As shown in Fig. 3 (MRM chromatograms of real samples ((a) sample A, (b) sample B)),peaks of chloramphenicol were found,however, the amountswere lower than the LLOQ for all samples. Compared with conventional off-line SPE,sample pretreatment was minimized and throughput of analysis was higher.

|
Download:
|
| Fig. 2. MRM chromatograms of chloramphenicol ((a) 20 pg/mL,spiked,(b) 2000 pg/ mL,spiked). | |
| Table 1 Intra-day precision and accuracy. |
| Table 2 Inter-day precision and accuracy. Concentration (pg/mL) 20 |

|
Download:
|
| Fig. 3. MRM chromatograms of real samples ((a) sample A,(b) sample B). | |
An on-line pretreatment LC-MS/MS method for analysis of chloramphenicol in honey was developed. Sugars were quickly and successfully removed with the methylcellulose-immobilized reversed-phase column. Fast extraction and analysis of chloramphenicol in honey were performed in 5 min. Results show that the method is reliable,reproducible and meets the requirement for the EU regulation of 0.3 mg/kg honey.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21227006) and the Research Fund for the Doctoral Program of Higher Education (No. 20110002110052).
| [1] | Commission Decision 2003/181/CE of 13 March 2003, Amending decision 2002/ 657/EC as regards the setting of minimum required performance limits (MRPLs) for certain residues in food of animal origin, Off. J. Eur. Commun. L71 (2003) 17-18. |
| [2] | M.I. Lopez, J.S. Pettis, I.B. Smith, P.S. Chu, Multiclass determination and confirmation of antibiotic residues in honey using LC-MS/MS, J. Agric. Food Chem. 56 (2008) 1553-1559. |
| [3] | R. Sheridan, B. Policastro, S. Thomas, D. Rice, Analysis and occurrence of 14 sulfonamide antibacterials and chloramphenicol in honey by solid-phase extraction followed by LC/MS/MS analysis, J. Agric. Food Chem. 56 (2008) 3509-3516. |
| [4] | É. Alechaga, E. Moyano, M.T. Galceran, Ultra-high performance liquid chromatography- tandem mass spectrometry for the analysis of phenicol drugs and florfenicol- amine in foods, Analyst 137 (2012) 2486-2494. |
| [5] | M. Liu, Y. Hashi, F. Pan, et al., Automated on-line liquid chromatography-photodiode array-mass spectrometry method with dilution line for the determination of bisphenol A and 4-octylphenol in serum, J. Chromatogr. A 1133 (2006) 142-148. |
| [6] | M. Liu, W. Yan, J.M. Lin, et al., On-line liquid chromatography-mass spectrometry with dilution line to achieve large volume urine injection for the improvement of sensitivity, J. Chromatogr. A 1198/1199 (2008) 87-94. |
| [7] | Y. Hashi, J.G. Yao, Y. Li, Y. Liu, J.M. Lin, Development of an on-line sample pretreatment liquid chromatography-mass spectrometry system for the identification of compounds in natural products, Chromatographia 67 (2008) 773-776. |
| [8] | W. Yan, Y. Li, L. Zhao, J.M. Lin, Determination of estrogens and bisphenol A in bovine milk by automated on-line C30 solid-phase extraction coupled with highperformance liquid chromatography-mass spectrometry, J. Chromatogr. A 1216 (2009) 7539-7545. |
| [9] | X. Lin, H. Li, X. He, et al., Automated on-line solid phase extraction coupled with high performance liquid chromatography-mass spectrometry for determination of deca-bromodiphenyl ether in human serum, J. Sep. Sci. 35 (2012) 2553-2558. |
| [10] | E. Yamamoto, K. Murata, Y. Ishihama, N. Asakawa, Methylcellulose-immobilized reversed-phase precolumn for direct analysis of drugs in plasma by HPLC, Anal. Sci. 17 (2001) 1155-1159. |
| [11] | S. Kawano, H. Murakita, E. Yamamoto, N. Asakawa, Direct analysis of drugs in plasma by column-switching liquid chromatography-mass spectrometry using a methylcellulose-immobilized reversed-phase pretreatment column, J. Chromatogr. B 792 (2003) 49-54. |
| [12] | S. Kawano, Y. Inohana, Y. Hashi, J.M. Lin, Analysis of keto-enol tautomers of curcumin by liquid chromatography/mass spectrometry, Chin. Chem. Lett. 24 (2013) 685-687. |






