In recent years,the solid-state materials possessing distinctive dielectric properties have triggered tremendous interests and attention owing to their potential applications in numerous fields, such as solid-state transducer,microelectronic devices and other components in microwave communication system [1, 2, 3, 4, 5]. However, existing researches have proven that only a limited number of inorganic materials have been discovered to meet the application types,which display the stringent permittivity requirements including heat loss,required power levels and operating electric field frequencies. Therefore,the research and exploration for ideal dielectric materials have hitherto remained a significant challenge [6, 7].
It is known to us that hybrid organic-inorganic complexes possessing the advantageous properties of both organic and inorganic compounds can show fascinating structures and excellent properties [8, 9, 10, 11, 12]. In organic-inorganic compounds,crown ethers have been the subject of various studies and occupied a special position,which is not only because of their extensive utilization for constructing the complex superstructures in supramolecular chemistry,but also for their abilities to form stable complexes with alkali and transition-metal ions and organic cations via hydrogen bonds due to their the modest flexibilities [13, 14, 15, 16, 17, 18, 19]. Especially,crown ethers act as ideal candidates for the formation of molecular stators for the design of phase change materials and ferroelectric molecular materials [20, 21]. Additionally, the supramolecular structures can be constructed through multiform weak intermolecular interactions,such as hydrogen bonding,charge transfer and van der Waals forces [22, 23, 24]. For example,a large number of novel supramolecular structures based on 18-crown-6 macrocyclic ether can afford multiple N-H···O strong H-bonds which contribute to the existence of dielectric behaviors. Besides,in the field of molecular machine design,the crown ethers often act as good molecular stators and can easily anchor the protonated R-NH3+ cation (R = aryl ring) into the cavity of crown ethers. The utilization of the R group as a molecular rotor or pendulum unit is expected to create desirable properties. To date,numerous derivatives and complexes based on crown ethers have been synthesized and characterized [23, 25, 26]. For instance, our group have successfully introduced 4-methoxyanilinium into the cavity of 18-crown-6 and obtained a novel supramolecular bola-like second-order ferroelectric phase-transition material [1, 2, 7, 8, 9].
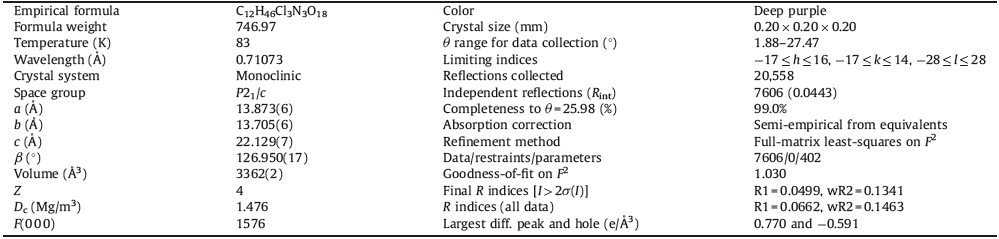
It was also suggested that compounds containing perchlorate and various aromatic amines can exhibit both ferroelectric and nonlinear optical physical properties. Perchlorate ion,ClO4-,is similar to BF4-. Because of its moderate size and highly symmetric shape,it is a stable anion and is generally accepted to be able to change the position easily with the varied temperature and weak interactions in crystals. In fact,for several ionic compounds we have studied their order-disorder transitions such as pyridium [27],methylammonium [28],and tetramethylammonium cations [29]. Taking these considerations into account,as a continuation of our systematic studies of phase transition [1, 2, 7, 8, 9, 30, 31],we herein report the synthesis (as shown in Scheme 1),crystal structure,dielectric properties of a new supramolecular macrocyclic compound [(NDPA)·(18-crown-6)]2+·(DMA)+·3ClO4- (1).
|
Download:
|
| Scheme 1. Synthesis process and structural diagram. | |
In this work,chemicals and solvents were commercially obtained as chemically pure and used without any further purification. Infrared spectra were taken with a Bruker Vector 22 spectrophotometer as KBr pellets in the 4000-400 cm-1 regions. Powder X-ray diffractionmeasurementswere madewith a Rigaku/D-MAXdiffraction system,equippedwith a copper X-ray tube (λCu-Κa = 1.5406 Å and a graphite monochromator. The powder patterns reported are the result of addition of three single scan patterns from 2θ = 5° to 2θ = 50° at 5 min-1 with an increment of 0.02°. Single-crystal datawere collected at 83 Kwith a Rigaku SCXmini CCD diffractometer equipped with graphitemonochromatedMo- Κa radiation. Dielectric studies (capacitance and dielectric-loss measurements) were performed on powder samples which had been pressed into tablets,on the surfaces of which conducting carbon glue was deposited. An automatic impedance TongHui2828 Analyzer was used. In the measured temperature range (120-420),the title structure showed no dielectric anomaly.
2.2. Synthesis of compound (1)NDPA (0.3 g,2.2 mmol) was dissolved in DMF (20 mL),to which HClO4 in water (70%,w/w) was added dropwise under stirring. Afterwards,the 18-crown-6 (0.58 g,2.2 mmol) water solution were mixed together. The mixed solution was heated to 120 ℃ for 4 h,and then cooled slowly to room temperature. Single crystals of 1 suitable for X-ray diffraction analysis were obtained via slow evaporation from the mixed solution at room temperature over two week. The crystals were deep purple,of block habit.
2.3. Single-crystal X-ray diffraction measurementSingle-crystal data collection of 1 was performed on a Rigaku SCXmini CCD diffractometer with Mo Κa radiation (l = 0.71073Å ) at 83 K. Structures of the complexes were determined by the direct methods routines in the SHELXS program and refined by fullmatrix least squares methods on F2 in SHELXL [32, 33, 34]. Hydrogen atoms bonded to the carbon atoms were placed in calculated positions and refined as riding mode,with C-H = 0.93Å (aromatic) and 0.96Å (methyl) with Uiso(H) = 1.2 Ueq(C). H atoms of amino groups were located in difference Fourier maps and in the last stage of refinement they were treated as riding on the N atom. Details about the data collection and refinement of complex 1 are summarized in Table 1,while selected bond distances,angles and H-bonding are given in Tables S1 and S2 in Supporting information.
| Table 1 Crystal data and structure refinement for compound 1. |
Supramolecular macrocyclic compound 1 (Scheme 1) was readily obtained as deep purple block crystals by slow evaporation of the solution at room temperature. If DMF was not added,there will no crystals be obtained. Firstly,the DMF was decomposed at 120 ℃,and formic acid and dimethylamine (DMA) were obtained. Then the dimethylamine (DMA) was protonated to form DMA cations. Finally,the cocrystals were formed though the deposition of DMA cations together with crown ethers and NDPA cations.
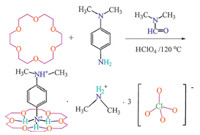
3.2. Spectral propertiesThe structure of 1 was characterized by IR and XRPD [35]. The IR spectra shown a series of strong peaks at 1465,1381,1105 and 721 cm-1,those peaks are the characteristic C-C vibration of aromatic ring. Moreover,the sharp peak at 2910 cm-1 corresponds to the aromatic C-H vibration. There is a broad band approximately 3450-3350 cm-1 it can suggest that the nitrogen atoms of the amines were protonated to formstrong H-bonds. The characteristic peaks of crown ether are shown in the region of 1410-1110 cm-1. In the range of 1150-1000 cm-1,we can see the characteristic peaks of ClO4- (Fig. S1 in Supporting information). The above spectral analyses match well with the determined single-crystal structure. The powder XRD pattern of 1 at room temperature matches very well with the pattern simulated from the single-crystal structure, which indicates the phasepurity of 1 (Fig. 1). Because of the different powder size during collection of the experimental XRPD data,there are some differences in reflection intensities between the simulated and experimental patterns.
|
Download:
|
| Fig. 1. The XRPD curve for compound 1. | |
Single-crystal X-ray analysis reveals that complex 1 crystallized in the monoclinic system P21/c with lattice parameters a = 13.873(6)Å ,b = 13.705(6)Å ,c = 22.129(7)Å ,b = 126.950(17)8 and V = 3362(2)Å 3.
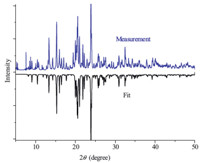
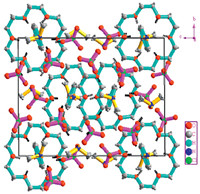
The asymmetric unit of 1 consists of 1:1 [(NDPA) (18-crown- 6)]2+ adduct in the supramolecular cation,one (DMA)+,three ClO4- anions. The molecular structure of 1 with the atomic label is shown in Fig. 2. The supramolecular [(NDPA)·(18-crown-6)]2+ cations and (DMA)+ cations are paired in this structure with the three relatively compact ClO4- anions. The ClO4- anion adopts a modestly flattened tetrahedron with four O which has been seen in many crystal structures. The packing diagram of complex 1 viewed along the a-axis is exhibited in Fig. 3. In the supramolecular cation of 1,the ammonium nitrogen atom is in the perching position,lying 0.6464Å away from the plane of the O18-crown-6 atoms of the crown ring. The supramolecular cation is constructed by six strong N- H· · ·O hydrogen bonds,in which the hydrogen atoms of the -NH3+ moiety are involved in bifurcated hydrogen bonds,each to two adjacent oxygen atoms of the crown ring,with the hydrogen bond lengths from 2.811(3)Å to 2.915(2)Å .
|
Download:
|
| Fig. 2. The asymmetric unit of 1,showing the atom-numbering scheme at 83 K. Dashed lines indicate hydrogen bonds. | |
|
Download:
|
| Fig. 3. View of the packing of the complex 1 (viewed along the a-axis). Dashed line indicated hydrogen bonds. | |
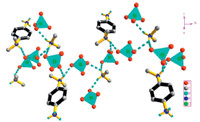
In the inorganic section,a charge consideration reveals that the species involved are three perchlorate anions. The Cl-O bond lengths (1.426(2)-1.4570(18)Å ) confirm the existence of flattened tetrahedron. More importantly,NDPA and DMA are protonated to form the H-bonding donors. Besides,three HClO4 are also deprotonated to act as the H-bonding acceptor. However,it is worthy to note that the perchlorates are linked to form onedimensional chain through strong N-H· · ·O hydrogen bonds along b-axis with the values of from 2.811(3)Å to 3.152(3)Å (Fig. 4).
|
Download:
|
| Fig. 4. View of the packing of the inorganic section (parallel to the b-axis). Dashed lines indicate the H-bonds. | |
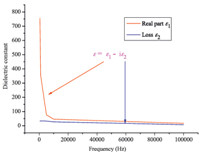
The measurement of dielectric constant for compound 1 (ε = ε1 - iε2,in which ε1 and ε2 are the corresponding real and imaginary parts of dielectric constant) was performed from 200 Hz to 1 MHz at 298 K. We can get a great deal of information on molecular motions from the frequency- and temperature-dependent dielectric constant. With increasing frequency the ε1 values of compound 1 decrease sharply and attain saturation at higher frequencies. At low frequencies,the high dielectric constant value is attributed to space charge polarization while the dipole reversal of space charge polarization cannot keep up the alternating current field reversal in the higher frequencies. That is the best explanation for phenomenon that the ε1 values become gradually smaller with the frequency increasing and tend to remain unchanged at higher frequencies. Obviously,the dielectric constant (ε = ε1 - iε2) vs. frequency measurement of 1 represents that the real part (ε1) of the dielectric constant decreases with an increase in the frequency (Fig. 5) at 298 K. It is interesting to note that the ε1 values of compound 1 at 200 Hz reaches a maximum value ca. 756.7,which sharply drops to ca. 16.9 at a relatively higher frequency of 1 MHz. The relative displacement of positive and negative ions and the turned polarization of molecular electric moment are the two main factors to determine the dielectric constant of 1.
|
Download:
|
| Fig. 5. The frequency-dependent dielectric constant of 1 at 1 MHz. | |
The ε1 and dielectric loss ε2 of compound of 1 were also measured in the temperature range of 120-420 K at 1 MHz (Fig. 6). The temperature-dependent dielectric constant of 1 shows no distinct anomalies at 1 MHz only with a gradually increase from 6.2 to 11.9 within the measured temperature range,together with the dielectric loss ε2 (from 0.10 to 0.47). This is probably owing to the freezing of the molecular rotation with the decreasing of temperature in the measured temperature range. At a relatively high temperature range,the freezing of the molecular rotator would be become activated for 1.

|
Download:
|
| Fig. 6. The temperature-dependent dielectric constant of 1 at 1 MHz. | |
The organic-inorganic hybrid supramolecular macrocyclic compound [(NDPA)·(18-crown-6)]2+·(DMA)+·3ClO4- has been synthesized and structurally characterized. In this structure,the NDPA combine with 18-crown-6 to constitute the supramolecular assembly through N-H· · ·O hydrogen bonds. Besides,the organic sheet and inorganic sheet are arranged alternately and also linked by N-O· · ·Hhydrogen bonds. More importantly,it is worthy to note that the perchlorates are linked to one-dimensional chain through strong N-H· · ·O hydrogen bonds along b-axis. Frequency-dependent dielectric constant of 1 at room temperature suggests that the relative displacement of ions and the turned polarization of molecular electric moment are the two main factors to determine the trend of dielectric constant. There is no distinguished dielectric anomaly observed in the temperature from 120 K to 420 K, probably because the molecular rotation is frozen in the measured temperature range.
AcknowledgmentsThis work was financially supported by the 973 Project (No. 2014CB848800),National Natural Science Foundation of China (Nos. 21422101 and 21301029),Jiangsu Province NSF (Nos. BK20140056 and BK20130600),Program for NCET and Ph.D. Programs Foundation of Ministry of Education of China (No. 20130092120013).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.10.005.
| [1] | D.W. Fu, H.L. Cai, W. Zhang, et al., Diisopropylammonium bromide is a hightemperature molecular ferroelectric crystal, Science 339 (2013) 425-428. |
| [2] | D.W. Fu, H.L. Cai, S.H. Li, et al., 4-Methoxyanilinium perrhenate 18-crown-6: a new ferroelectric with order originating in swinglike motion slowing down, Phys. Rev. Lett. 110 (2013) 257601. |
| [3] | W. Zhang, R.G. Xiong, Ferroelectric metal-organic frameworks, Chem. Rev. 112 (2012) 1163-1195. |
| [4] | Z.H. Sun, X.Q. Wang, J.H. Luo, et al., Ferroelastic phase transition and switchable dielectric behavior associated with ordering of molecular motion in a perovskitelike architectured supramolecular cocrystal, J. Mater. Chem. C 1 (2013) 2561-2567. |
| [5] | H.N. Lee, H.M. Christen, M.F. Christen, C.M. Rouleau, D.H. Lowndes, Strong polarization enhancement in asymmetric three-component ferroelectric superlattices, Nature 433 (2005) 395-399. |
| [6] | H.Y. Ye, D.W. Fu, Y. Zhang, et al., Hydrogen-bonded ferroelectrics based on metal-organic coordination, J. Am. Chem. Soc. 131 (2009) 42-43. |
| [7] | D.W. Fu, Y.M. Song, G.X. Wang, et al., Dielectric anisotropy of a homochiral trinuclear nickel(Ⅱ) complex, J. Am. Chem. Soc. 129 (2007) 5346-5347. |
| [8] | D.W. Fu, W. Zhang, Y. Zhang, et al., Supramolecular bola-like ferroelectric: 4- methoxyanilinium tetrafluoroborate-18-crown-6, J. Am. Chem. Soc. 133 (2011) 12780-12786. |
| [9] | D.W. Fu, W. Zhang, H.L. Cai, et al., Diisopropylammonium chloride: a ferroelectric organic salt with a high phase transition temperature and practical utilization level of spontaneous polarization, Adv. Mater. 23 (2011) 5658-5662. |
| [10] | H. Hughes, M.M.B. Allix, C.A. Bridges, et al., A polar oxide with a large magnetization synthesized at ambient pressure, J. Am. Chem. Soc. 127 (2005) 13790-13791. |
| [11] | T. Akutagawa, H. Koshinaka, D. Stao, et al., Ferroelectricity and polarity control in solid-state flip-flop supramolecular rotators, Nat. Mater. 8 (2009) 342-347. |
| [12] | Y.E. Alexeev, B.I. Kharisov, T.C. Hermández García, A.D. Garnovskii, Coordination motifs in modern supramolecular chemistry, Coord. Chem. Rev. 254 (2010) 794- 831. |
| [13] | G.W. Gokel, W.M. Leevy, M.E. Weber, Crown ether: sensors for ions and molecular scaffolds for materials and biological models, Chem. Rev. 104 (2004) 2723-2750. |
| [14] | D.W. Fu, W. Zhang, R.G. Xiong, et al., A multiferroic perdeutero metal-organic framework, Angew. Chem. Int. Ed. 50 (2011) 11947-11951. |
| [15] | D.W. Fu, Y. Zhang, H.L. Cai, et al., The first example of a molecular-based ferroelectric with barium cation: catena-(mu(2)-nitrite-O,O)-bi-aqua-(18- crown-6)-barium nitrite, J. Mater. Chem. 22 (2012) 17525-17530. |
| [16] | D. Braga, M. Gandolfi, M. Lusi, et al., Solution and solid-state preparation of 18- crown [6] complexes with M[HSO4] (n) salts (M = NH4+, K+, Sr2+ and n = 1,2) and an investigation of solvation/desolvation processes and crystal polymorphism, Chem. Eur. J. 13 (2007) 5249-5255. |
| [17] | P.C. Junk, B.J. McCool, B. Moubaraki, et al., Utilization of crown ethers to stabilize the dinuclear μ-oxo bridged iron (Ⅲ) aqua ion, [(H2O)5Fe(mu-O) Fe(OH2)5]4+, J. Chem. Dalton Trans. 6 (2002) 1024-1029. |
| [18] | J.M. Harrington, S.B. Jones, P.H. White, R.D. Hancock, Possible role of relativistic effects in the plasticity of the coordination geometry of Cadmium (Ⅱ). A voltammetric study of the stability of the complexes of cadmium (Ⅱ) with 12-crown-4, 15-crown-5 and 18-crown-6 in aqueous solution and the structures of [Cd(benzo- 18-crown-6)(NCS)2] and [K(18-crown-6)][Cd(SCN)3], Inorg. Chem. 43 (2004) 4456-4463. |
| [19] | J.M. Dou, X. Gao, F.Y. Dong, D.C. Li, D.Q. Wang, One-or two dimensional 2,3- naphtho crown ether complexes [Na(N15C15)]2[M(SCN)4] and [K(N18C6)]2[M(SCN)4] (M = Pd, Pt) constructed by pi-pi stacking interactions, Dalton Trans. 18 (2004) 2918-2922. |
| [20] | D. Braga, M. Gandolfi, M. Lusi, et al., Solution and solid-state preparation of 18- crown-6 and 15-crown-5 adducts of hydrogen sulfate salts and an investigation of the reversible dehydration processes, Cryst. Growth Des. 7 (2007) 919-924. |
| [21] | D. Braga, M. Curzi, M. Lusi, F. Grepioni, Unprecedented mechanochemical preparation of 18Crown[6] and 15Crown[5] adducts of ammonium hydrogen sulfate by grinding or kneading, CrystEngComm 7 (2005) 276-278. |
| [22] | R.M. Izatt, J.C. Bradshaw, S.A. Nielsen, et al., Thermodynamic and kinetic data for cation macrocycle interaction, Chem. Rev. 85 (1985) 271-339. |
| [23] | W.S. You, E.B. Wang, L. Xu, et al., Synthesis and structural characterization of a new supramolecular compound: H3PW12O40·6C14H20O5·16H2O (C14H20O5 = benzo-15-crown-5), J. Mol. Struct. 554 (2000) 141-147. |
| [24] | H.W. Gibson, N. Yamaguchi, L. Hamilton, J.W. Jones, Cooperative self-assembly of dendrimers via pseudorotaxane formation from a homotritopic guest molecular and complementary monotopic host dendrons, J. Am. Chem. Soc. 124 (2002) 4653-4665. |
| [25] | D. Braga, E. Modena, M. Polito, K. Rubini, F. Grepioni, Crystal forms of highly "dynamic" 18-crown[6] complexes with M[HSO4] and M[H2PO4] (M+= NH4+, Rb+, Cs+): thermal behaviour and solid-state preparation, New J. Chem. 32 (2008) 1718-1724. |
| [26] | S.G. Li, J.H. Luo, Z.H. Sun, et al., Phase transition triggered by ordering of unique pendulum-like motions in a supramolecular complex: potassium hydrogen bis(- dichloroacetate)-18-crown-6, Cryst. Growth Des. 13 (2013) 2675-2679. |
| [27] | P. Czarnecki, A. Katrusiak, I. Szafraniak, J. Wasicki, Experimental evidence for a continuous phase transition in a multidimensional ferroelectric, Phys. Rev. B 57 (1998) 3326-3332. |
| [28] | N. Onoda Yamamuro, O. Yamamuro, T. Matsuo, H. Suga, Heat capacities and phase transition of protonated and deuterated methylammonium tetrafluoroborates, J. Phys. Chem. 100 (1996) 19647-19652. |
| [29] | E. Palacios, J.J. Melero, R. Burriel, P. Ferloni, Structual, calorimetric, and Monte Carlo investigation of the order-disorder transition of BF4 in (CH3)4NBF4, Phys. Rev. B 54 (1996) 9099-9108. |
| [30] | T. Akutagawa, D. Endo, F. Kudo, et al., A solid-state supramolecular rotator assenbled from a Cs-crown ether polyoxometalate hybrid: (Cs+)3([18]crown- 6)3(H+)2[PMo12O40], Cryst. Growth Des. 8 (2008) 812-816. |
| [31] | D.H. Wu, J.Z. Ge, H.L. Cai, W. Zhang, R.G. Xiong, Organic salt of hydrogen L-tartaric acid: a novel wide-temperature-range ferroelectrics with a reversible phase transition, CrystEngComm 13 (2011) 319-324. |
| [32] | SAINT-Plus, version 6.02, Bruker Analytical X-ray System, Madison, WI, 1999. |
| [33] | G.M. Sheldrick, SADABSs: An Empirical Absorption Correction Program, Bruker Analytical X-ray Systems, Madison, WI, 1996. |
| [34] | G.M. Sheldrick, SHELXTL-97, Universität of Göttingen, Göttingen, Germany, 1997. |
| [35] | K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd ed., Wiley, New York, 1978. |