Pyridone and pyran structural units are widely occurring in various molecules exhibiting a wide range of biological activities and serve as a specific nonnucleoside reverse transcriptase inhibitor of HIV-1 [1, 2],inotropic and vasodilatatory drugs [3], antitumors and antioxidants [4, 5],rhinovirus 3C protease inhibitors [6],anticancers [5, 7],potential antiviral and antileishmanial agents [8]. Also compounds containing a 2-pyridone moiety fused with a substituted pyran ring are reported as a Ca2+ inhibitor [9], active against multidrug resistant KB-VI cancer cells and a selective cytotoxicity profile [10]. Therefore,a variety of synthetic strategies have been developed for the preparation of dihydropyrano[4,3-b] pyran derivatives that often proceeds through the formation of the intermediate Knoevenagel products and their subsequent reactions with 4-hydroxy-6-methylpyran-2-one [11] or a multicomponent reaction of pyrone with malononitrile and various aromatic aldehydes [9].
Homogeneous inorganic acids and alkali such as sulfuric acid, potassium hydroxide and sodium hydroxide can act as the catalysts in organic transformations. However,these catalysts have some disadvantages: they are strongly corrosive and nonrenewable and may easily cause environmental pollution through wastewater or sludge discharging. Solid acids as economic and ecologically benign catalysts have offered unique properties and important advantages over the homogeneous inorganic acids in organic synthesis in recent years; for example,operational simplicity,environmental compatibility,nontoxic,low cost,and ease of isolation [10, 11, 12, 13, 14, 15]. However,they have some disadvantages, for example,although zeolites demonstrate higher activity,their reactions typically give a variety of undesired by-products due to the higher temperatures employed and metal triflates are costly and moisture sensitive and also some of the catalysts require the special efforts to prepare [16]. Ion exchange resins are limited in application because they are thermally unstable above 120 ℃ in the acid form [17].
Green chemistry with its 12 principles would like to increases the efficiency of synthetic methods,to use less toxic solvents, reduce the stages of the synthetic routes and minimize waste as far as practically possible [18]. One of the key areas of green chemistry is the replacement of hazardous solvents with environmentally benign ones or the elimination of solvents altogether [18, 19, 20]. By changing the methodologies of organic synthesis health and safety will be advanced in the small scale laboratory level but also will be extended to the industrial large scale production processes through the new techniques. Another beneficiary of course will be the environment through the use of less toxic reagents, minimization of waste and more biodegradable by-products [21, 22, 23].
Recently,succinimide sulfonic acid was synthesized and their application in the variety of organic transformations was investigated [24]. Herein,a new BrÖnsted acid,namely,4- (succinimido)-1-butane sulfonic acid (SBSA) is introduced and its application in the promotion of the synthesis of dihydropyrano[ 4,3-b]pyran derivatives is described. The present study is developed as a new preparative procedure for this class of heterocyclic scaffolds by utilizing SBSA under solvent-free conditions.
2. Experimental 2.1. GeneralChemicals were purchased from Fluka AG,Merck and Synthetic Chemicals Ltd. Reaction monitoring and purity determination of the products were accomplished by TLC or GC-MS on an Agilent GC-Mass-6890 instrument under 70 eV conditions. IR and FTIR Spectra were obtained using a Perkin-Elmer spectrometer 781 and Bruker Equinox 55 using KBr pellets for solid and neat for liquid samples in the range of 4000-400 cm-1. In all the cases the 1HNMR spectra were recorded with Bruker Avance 400 MHz instrument using. Mass spectra were recorded with PESciex model API 3000 instrument. Microanalyses were performed on a Perkin-Elmer 240-B microanalyzer. Melting points were recorded on a Bu¨ chi B- 545 apparatus in open capillary tubes.
2.2. Synthesis of 4-(succinimido)-1-butane sulfonic acid (SBSA)Succinimide (0.99 g,10 mmol) was added to 1,4-butane sultone (1.5 mL 14.4 mmol) and stirred continuously for 10 h at 40-50 ℃ by using solar energy to obtain 4-(succinimio)-1-butane sulfonic acid as a white solid. The viscous liquid was washed by diethyl ether for three times to remove any unreacted starting materials, and then a white solid was obtained. The resulting SBSA was dried to constant weight in vacuum at 60 ℃. The white needles were obtained by crystallization in a mixture of ethanol and water using slow evaporation technique (2.12 g,yield 90.2%). Mp 222 ℃ (dec.); IR (KBr,cm-1): νmax 3140,3090,2980,2940,1740,1640,1600, 1460,1380,1190,1120,1040; 1H NMR (300 MHz,D2O): δ 1.75- 1.68 (m,2H,-CH2-),2.03-1.91 (m,2H,-CH2-),2.64 (s,4H,-CH2- CH2-,succinimide),2.95 (t,2H,J = 7.4 Hz,-CH2-S),4.23 (t,2H, J = 6.9 Hz,-CH2-N); 13C NMR (75 MHz,D2O): δ 22.3 (2 of butane), 28.2 (C3 of butane),29.3 (CH2 of succinimide),49.3 (N-CH2),51.2 (S-CH2),186.5 (C=O).
2.3. The preparation of 2-amino-4-aryl-7-methyl-5-oxo-4,5-dihydropyrano[4,3-b]pyran-3-carbonitriles (2)In a 25 mL round bottom flask a mixture of 4-hydroxy-6- methylpyran-2-one (1.0 mmol),aromatic aldehyde (1.0 mmol), malononitrile (1.0 mmol) were mixed in presence of 4-(succinimido)- 1-butane sulfonic acid (10 mg) at 60 ℃ under solvent-free condition for appropriate time. After completion of the reaction (monitored by TLC),the reaction mixture was cooled to room temperature and water was added and the solid precipitated was filtered to separate the catalyst. Water was evaporated under reduced pressure and the catalyst was recovered and used for the next run. The solid product was recrystallized from ethanol to yield the pure product.
2-Amino-4-(4-fluorophenyl)-7-methyl-5-oxo-4,5-dihydropyrano[ 4,3-b]pyran-3-carbonitrile (2c): Colorless solid; mp 221- 223 ℃; IR (KBr,cm-1): νmax 3369,3317,3195,2924,2194, 1715,1678,1641,1618,1591,1378,1259,1138,1091,1032,978; 1H NMR (400 MHz,DMSO-d6): δ 2.19 (s,3H,CH3),4.28 (s,1H,CH), 6.31 (s,1H,CH),7.19 (brs,2H,NH2),7.19-7.22 (m,2H,ArH),7.31- 7.34 (m,2H,ArH).
2-Amino-4-(4-bromophenyl)-7-methyl-5-oxo-4,5-dihydropyrano[ 4,3-b]pyran-3-carbonitrile (2d): Colorless solid; mp 225- 227 ℃; IR (KBr,cm-1): νmax 3381,3322,3197,2921,2204,1712, 1676,1643,1611,1596,1384,1263,1141,1095,1036,972; 1H NMR(400 MHz,DMSO-d6): δ 2.21 (s,3H,CH3),4.31 (s,1H,CH),6.27 (s,1H,CH),7.18 (d,2H,J = 8.0 Hz,ArH),7.25 (s,2H,NH2),7.46 (d, 2H,J = 8.0 Hz,ArH).
4,40-(1,4-Phenylene)bis(2-amino-7-methyl-5-oxo-4,5-dihydropyrano[ 4,3-b]pyran-3-carbonitrile) (2m): Colorless solid; mp 256-258 ℃; IR (KBr,cm-1): νmax 3372,3317,3196,2196,1699, 1673,1614,1463,1383; 1H NMR (400 MHz,DMSO-d6): δ 2.16 (s, 6H,2CH3),4.19 (s,2H,2CH),6.22 (s,2H,2CH),7.06 (s,4H,Ar-H), 7.13 (brs,4H,2NH2); 13C NMR (100 MHz,DMSO-d6): δ 18.9,35.5, 57.8,98.0,119.4,127.5,130.1,136.6,142.4,158.6,161.2,161.9, 162.8,174.8; MS (ESI): m/z [M+1]+ 483; Anal. Calcd. for C26H18 N4O6: C,64.73; H,3.73; N,11.62%. Found: C,64.62; H,3.83; N, 11.78%.
3. Results and discussionPart of our research is aiming to introduce the eco-efficient methodology that allows decreasing the amount of waste and a lesser use of hazardous materials is proposed. In preparation of succinimide-N-sulfonic acid,chlorosulfonic acid was stirred with succinimide to generate gaseous HCl [24]. However,it has the disadvantage of using chlorosulfonic acid which causes severe burns and reacts exothermically and violently with water producing sulfuric acid,hydrochloric acid,and large quantities of dense white acid fumes. Also it is very toxic by inhalation and corrosive to metals. The present catalyst was prepared by mixing succinimide and 1,4-butane sultone that is more simple and safer. The synthesis of 4-(succinimido)-1-sulfonic acid involved stirring same equivalents of succinimide and 1,4-butane sultone at 40- 50 ℃ for 6 h. The present method does not use traditional heater. Instead,10 mirrors reflect the sunlight onto the 25 mL round bottom flask. When the concentrated sunlight strikes the round bottom flask,it heats the mixture of reaction to 40-60 ℃. The viscous liquid was washed by diethyl ether,and then a white solid was obtained. The resulting SBSA was dried to constant weight in vacuum. The structure was confirmed by IR,1H NMR,and 13C NMR. The content of water of SBSA was 5.4% using Karl-Fischer titration method. SBSA was soluble in DMSO,DMF,water,methanol and ethanol; however it was immiscible with diethyl ether,ethyl acetate,and dichloromethane. So the catalyst can be separated conveniently from products by simple phase separations (Scheme 1).

|
Download:
|
| Scheme 1. Synthesis of 4-(succinimido)-1-butane sulfonic acid (SBSA) by using solar energy. | |
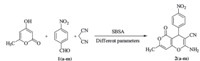
To evaluate the effect of the amount of SBSA,condensation of 4-hydroxy-6-methylpyran-2-one,4-nitrobenzaldehyde (1e) and malononitrile was carried out in presence of different amounts of (2.1%,4.2% and 8.5 mol%) under solvent-free conditions (Scheme 2). It was observed that 4.2 mol% of SBSA was an optimum amount for this model reaction to furnish the desired product in high yield. Increasing the amount of the catalyst beyond 4.2 mol% did not increase the yield noticeably. Also the different reaction temperatures (r.t. -80 ℃) were analyzed and the results showed that 88% of 2-amino-4-(4-nitrophenyl)-7-methyl-5-oxo- 4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile was offered in the presence of 4.2 mol% SBSA at 60 ℃ within 60 min. Higher temperatures caused more spots on the TLC and it seems that by-products were obtained. The reaction was not completed at 60 ℃ even after 4 h in absence of SBSA and only 38% of 2e was offered.
|
Download:
|
| Scheme 2. Synthesis of 2-amino-4-aryl-7-methyl-5-oxo-4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile derivatives in presence of SBSA under various reaction conditions. | |
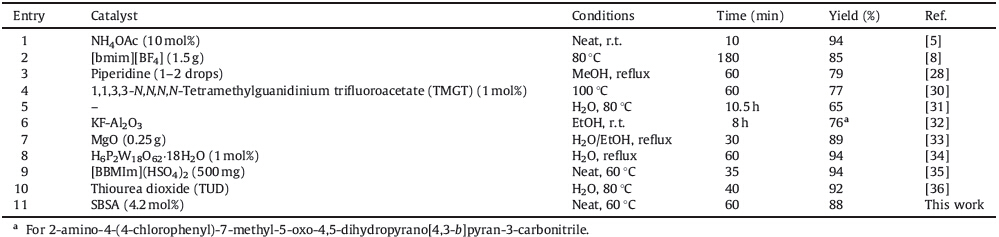
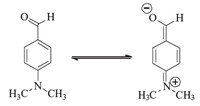
In order to evaluate the generality of this model reaction,a range of 2-amino-4-aryl-7-methyl-5-oxo-4,5-dihydropyrano[4,3- b]pyran-3-carbonitriles 2a-m were prepared under optimized reaction conditions in presence of SBSA (Table 1). The aryl aldehydes which possess electron-donating and electron-withdrawing substituents and heteryl aldehydes provided desired dihydropyrano[4,3-b]pyrans in good to high yields without involving any side products (Table 1,entries 1-13). However, aliphatic aldehydes did not undergo pyranization even within long reaction time and elevated temperature; TLC and GC-MS analysis of the reaction mixture showed numerous products. The electrondonating substituents caused lower yields and longer reaction times than electron-withdrawing substituents (Table 1,entries 5, 7,9 and 10). 4,4'-(1,4-Phenylene)bis(2-amino-7-methyl-5-oxo- 4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile) 2m was obtained in 80% yield when terephthaldehyde 1m was reacted with malononitrile and 4-hydroxy-6-methylpyran-2-one in molar ratio 1.0:2.0:2.0 under optimized reaction conditions (Table 1,entry 13). 4-Dimethylaminobenzaldehyde failed to give the corresponding pyran derivative and the starting materials were quantitatively recovered under the same conditions (Table 1,entry 14). The explanation for this result may be due to the strong electrondonating dimethylamino group which will reduce the reactivity. A degree of tautomerisation may occur with formation of a quinonoid structure as shown in Scheme 3 and thus decrease the reactivity of the aldehyde group [25].
| Table 1 Synthesis of 2-amino-4-aryl-7-methyl-5-oxo-4,5-dihydropyrano[4,3-b]pyran-3-carbonitriles in the presence of SBSA.a |
|
Download:
|
| Scheme 3. Tautomerisation with formation of a quinonoid structure. | |
New products were characterized by IR,1H NMR,13C NMR, MASS spectra and elemental analysis and known products were characterized by IR and 1H NMR and comparison of their melting points with those of authentic samples.
SBSA was isolated and could be recycled up to five times without any significant loss of activity (Table 1,entry 5). The proposed mechanism for the formation of the product via tandem Knoevenagel-cyclo condensation is outlined in Scheme 4. Carbonyl group of aldehyde (1) was activated by BrÖnsted acid SBSA. Next, nucleophilic attack of the malononitril on the carbonyl carbon was caused to form intermediate arylidene malononitrile. Subsequent Michael addition of 4-hydroxy-6-methylpyran-2-one followed by cyclization afforded the product (2).

|
Download:
|
| Scheme 4. The proposed mechanism for the synthesis of 2-amino-4-aryl-7-methyl-5-oxo-4,5-dihydropyrano[4,3-b]pyran-3-carbonitriles in presence of [BBMIm](HSO4)2 at 60 ℃. | |
To validate the proposed mechanism,the synthesis of 2b was carried out in two steps. Firstly,4-chlorobenzylidene malononitrile was prepared by the condensation of 4-chlorobenzaldehyde 1b and malononitrile in presence of SBSA. Then product of the first step was reacted with 4-hydroxy-6-methylpyran-2-one in presence of SBSA to give the product 2b (84%) in 75 min. This fact provided the evidence in support of the intermediate arylidene malononitrile proposed pathway.
The comparison of the present methodology with previously reported procedures for the synthesis of 2a is shown in Table 2. As can be seen,the reaction catalyzed by SBSA at 60 ℃ give a comparable yield,requires less amount of catalyst and less time than other protocols and also it is reusable.
| Table 2 Comparison of the present method with other reported strategies for the synthesis of 2-amino-4-phenyl-7-methyl-5-oxo-4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile. |
In conclusion,a novel BrÖnsted acid is introduced and its catalytic activity was investigated for the synthesis of 2-amino-4- aryl-7-methyl-5-oxo-4,5-dihydropyrano[4,3-b]pyran-3-carbonitrile derivatives under solvent-free conditions. To prepare of the catalyst,solar energy was applied for the first time and hazardous material namely chlorosulfonic acid was avoided. The current method has the advantages of simple experimental procedure, good to high yield of products,and reusability of the catalyst.
AcknowledgmentThe author is thankful to the Research House of Professor Reza, Education Guilan,Rasht,Iran for the partial support of this work.
| [1] | E. De Clercq, Perspectives of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection, II Farmaco 54 (1999) 26-45. |
| [2] | R.L.T. Parreira, O. Abrahão, S.E. Galembeck, Conformational preferences of nonnucleoside HIV-1 reverse transcriptase inhibitors, Tetrahedron 57 (2001) 3243- 3253. |
| [3] | E.L. Presti, R. Boggia, A. Feltrin, et al., 3-Acetyl-5-acylpyridin-2(1H)-ones and 3- acetyl-7,8-dihydro-2,5(1H,6H) quinolinediones: synthesis, cardiotonic activity and computational studies, II Farmaco 54 (1999) 465-474. |
| [4] | W.K. Anderson, D.C. Dean, T. Endo, Synthesis, chemistry, and antineoplastic activity of a-halopyridinium salts: potential pyridone prodrugs of acylated vinylogous carbinolamine tumor inhibitors, J. Med. Chem. 33 (1990) 1667-1675. |
| [5] | D. Rajguru, B.S. Keshwal, S. Jain, Solvent-free, green and efficient synthesis of pyrano[4,3-b]pyrans by grinding and their biological evaluation as antitumor and antioxidant agents, Med. Chem. Res. 22 (2013) 5934-5939. |
| [6] | P.S. Dragovich, T.J. Prins, R. Zhou, et al., Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 6. Structure-activity studies of orally bioavailable, 2-pyridone-containing peptidomimetics, J. Med. Chem. 45 (2002) 1607-1623. |
| [7] | W. Kemnitzer, J. Drewe, S.C. Jiang, et al., Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 3. Structure-activity relationships of fused rings at the 7,8- positions, J. Med. Chem. 50 (2007) 2858-2864. |
| [8] | X. Fan, D. Feng, Y. Qu, et al., Practical and efficient synthesis of pyrano[3,2-c]pyridone, pyrano[4,3-b]pyran and their hybrids with nucleoside as potential antiviral and antileishmanial agents, Bioorg. Med. Chem. Lett. 20 (2010) 809- 813. |
| [9] | K. Tatsuta, T. Yamaguchi, Y. Tsuda, et al., The first total synthesis and structural determination of YCM1008A, Tetrahedron Lett. 48 (2007) 4187- 4190. |
| [10] | K. Tanabe, W.F. Hölderich, Industrial application of solid acid-base catalysts, Appl. Catal. A 181 (1999) 399-434. |
| [11] | D.J. Cole-Hamilton, Homogeneous catalysis - new approaches to catalyst separation, recovery, and recycling, Science 299 (2003) 1702-1706. |
| [12] | A. Chakrabarti, M.M. Sharma, Cationic ion exchange resins as catalyst, React. Polym. 20 (1993) 1-45. |
| [13] | J.M. Riego, Z. Sedin, J.M. Zaldivar, N.C. Marziano, C. Tortato, Sulfuric acid on silicagel: an inexpensive catalyst for aromatic nitration, Tetrahedron Lett. 37 (1996) 513-516. |
| [14] | N.J. Turro, Photochemistry of ketones adsorbed on porous silica, Tetrahedron 43 (1987) 1589-1616. |
| [15] | N.G. Khaligh, P.G. Ghasem-Abadi, N-Sulfonic acid poly(4-vinylpyridinum) hydrogen sulfate as a novel, efficient, and reusable solid acid catalyst for acylation under solventpfree conditions, Chin. J. Catal. 35 (2014) 1126-1135. |
| [16] | B.X. Du, M.Y. Yin, M.M. Zhang, Y.L. Li, X.S. Wang, Yb(OTf)3: an efficient catalyst for the synthesis of 11-aryl-7H-cyclopenta[b][4,7]phenanthrolin-10(11H)-one derivatives, J. Heterocycl. Chem. 49 (2012) 1439-1442. |
| [17] | A.D. Patil, A.J. Freyer, S.E. Drake, et al., The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn., J. Med. Chem. 36 (1993) 4131-4138. |
| [18] | P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998. |
| [19] | D. Warren, Green Chemistry. A Teaching Resource, Royal Society of Chemistry, Cambridge, 2001. |
| [20] | J. Clark, D. Macquarrie, Handbook of Green Chemistry and Technology, Blackwell Publishing, Abingdon, Oxfordshire, 2002. |
| [21] | M. Poliakoff, P. Licence, Sustainable technology: green chemistry, Nature 450 (2007) 810-812. |
| [22] | P. Tundo, P.T. Anastas (Eds.), Green Chemistry: Challenging Perspectives, Oxford University Press, Oxford, UK, 2000. |
| [23] | R.A. Sheldon, I. Arends, Green Chemistry and Catalysis, Wiley-VCH, Indianapolis, USA, 2006. |
| [24] | (a) F. Shirini, N.G. Khaligh, Succinimide-N-sulfonic acid: a mild, efficient, and reusable catalyst for the chemoselective trimethylsilylation of alcohols and phenols, Phosphorus Sulfur Silicon Relat. Elem. 186 (2011) 2156-2165; (b) F. Shirini, N.G. Khaligh, Succinimide-N-sulfonic acid: an efficient catalyst for the synthesis of xanthene derivatives under solvent-free conditions, Dyes Pigm. 95 (2012) 789-794; (c) F. Shirini, N.G. Khaligh, A succinimide-N-sulfonic acid catalyst for the acetylation reactions in absence of a solvent, Chin. J. Catal. 34 (2013) 695-703; (d) F. Shirini, N.G. Khaligh, Succinimide-N-sulfonic acid catalyzed synthesis of bis(indolyl)methane and coumarin derivatives under mild conditions, Chin. J. Catal. 34 (2013) 1890-1896. |
| [25] | T.S. Jin, G. Sun, Y.W. Li, T.S. Li, An efficient and convenient procedure for the preparation of 1,1-diacetates from aldehydes catalyzed by H2NSO3H, Green Chem. 4 (2002) 255-256. |
| [26] | M.Z. Piao, K. Imafuku, Convenient synthesis of amino-substituted pyranopyranones, Tetrahedron Lett. 38 (1997) 5301-5302. |
| [27] | I.V. Magedov, M. Manpadi, M.A. Ogasawara, et al., Structural simplification of bioactive natural products with multicomponent synthesis. 2. Antiproliferative and antitubulin activities of pyrano[3,2-c]pyridones and pyrano[3,2-c]quinolones, J. Med. Chem. 51 (2008) 2561-2570. |
| [28] | E.V. Stoyanov, I.C. Ivanov, D. Heber, General method for the preparation of substituted 2-amino-4H,5H-pyrano[4,3-b]pyran-5-ones and 2-amino-4H-pyrano[ 3,2-c]pyridine-5-ones, Molecules 5 (2000) 19-23. |
| [29] | D.Q. Shi, L.H. Niu, Q.Y. Zhuhang, Synthesis of pyrano[3,2-c]pyran-5-one derivatives by three-component one-pot reaction in aqueous media, Chin. J. Org. Chem. 28 (2008) 1633-1636. |
| [30] | A. Shaabani, S. Samadi, Z. Badri, A. Rahmati, Ionic liquid promoted efficient and rapid one-pot synthesis of pyran annulated heterocyclic systems, Catal. Lett. 104 (2005) 39-43. |
| [31] | A. Shaabani, S. Samadi, A. Rahmati, One-pot, three-component condensation reaction in water: an efficient and improved procedure for the synthesis of pyran annulated heterocyclic systems, Synth. Commun. 37 (2007) 491-499. |
| [32] | X.S. Wang, J.X. Zhou, Z.S. Zeng, et al., One-pot synthesis of pyrano[3,2-c]pyran derivatives catalyzed by KF/Al2O3, Arkivoc 11 (2006) 107-113. |
| [33] | M. Seifi,H. Sheibani, High surface areaMgOas a highly effective heterogeneous base catalyst for three-component synthesis of tetrahydrobenzopyran and 3,4-dihydropyrano[c]chromene derivatives in aqueous media, Catal. Lett. 126 (2008) 275-279. |
| [34] | D. Rajguru, B.S. Keshwal, S. Jain, H6P2W18O62 18H2O: a green and reusable catalyst for one-pot synthesis of pyrano[4,3-b]pyrans in water, Chin. Chem. Lett. 24 (2013) 1033-1036. |
| [35] | N.G. Khaligh, 1,10-Butylenebis(3-methyl-3H-imidazol-1-ium) hydrogensulfate as a halogen-free and reusable binuclear Brönsted ionic liquid catalyzed the synthesis of pyrano[4,3-b]pyran derivatives, Monatsh. Chem. 145 (2014) 1643-1648. |
| [36] | M. Ghashang, S.S. Mansoor, K. Aswin, Thiourea dioxide: an efficient and reusable organocatalyst for the rapid one-pot synthesis of pyrano[4,3-b]pyran derivatives in water, Chin. J. Catal. 35 (2014) 127-133. |