b Key Laboratory of Yangtze River Water Environment, Ministry of Education, Tongji University, Shanghai 200092, China
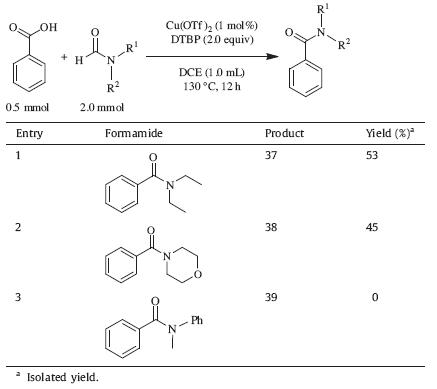
As one of the most common chemical linkages,the amide bond is ubiquitous in a broad range of organic molecules. By linking amino acids to form proteins,it represents the main chemical bonds in biological system and plays a crucial role in the sustenance of life [1]. Moreover,the amide bond is widely present in drug molecules,agrochemicals,materials,and other natural products [2]. Although a vast array of methods are available for the formation of amide bonds,the most common one involves the coupling reaction of activated carboxylic acids with amines [3]. For this method,carboxylic acids should be preactivated by transforming the hydroxyl groups of the acids into good leaving groups. Unfortunately,the activation of carboxylic acids requires the use of a stoichiometric amount of coupling reagents,and the reactions produce large quantities of potentially hazardous chemical wastes [4]. Due to the great drawbacks of the current methods and the importance of amide bonds,amide bond formation is still the subject of extensive studies and has been voted as the most important synthetic transformation to be improved by the American Chemical Society’s (ACS) Green Chemistry Institute (GCI) Pharmaceutical Roundtable (PR) in 2005 [5]. One obvious strategy to circumvent the problems of the current amide bond formation reactions is to use carboxylic acids as starting materials directly (Fig. 1). Although this strategy is expected to be very challenging,it has still attracted more and more attention due to its great advantages and should be extensively investigated [2d, 6].
While N,N-dimethylformamide (DMF) is primarily used as a polar solvent in chemical reactions,it is a multipurpose reagent and plays various roles in organic synthesis [7]. As a formamide containing an aldehyde and amino group,DMF can serve as a precursor to generate a wide range of intermediates in organic reactions,including -CONMe2,CHO,-NMe2,-CO,O,-Me,etc. [8]. Therefore,reactions involving DMF as the reaction precursor has gained considerable interest recently,and its various reactivities have been extensively exploited to develop a wide range of novel organic reactions,especially those enabled by transition metals [7, 8].
|
Download:
|
| Fig. 1. Two strategies for amide bond formation. | |
Herein,we report a copper-catalyzed amide bond formation reaction utilizing carboxylic acids and formamides as the amino source [9]. It should be mentioned that several amidation reactions were reported just when we almost finished this work. The Reddy group disclosed the coupling reaction of carboxylic acids and formamides using Cu(ClO4)2·6H2O (10 mol%) as catalyst,tert-butyl hydroperoxide (TBHP) (1.5 equiv.) as oxidant and DMF as the solvent and source of the amine [10]. Subsequently,the Song and Li group reported similar amide bond formation reactions under the conditions consisting of CuCl2 (5 mol%),1,4-diazabicyclo[2.2.2]octane (DABCO) (10 mol%),TBHP (2 equiv.),and DMF (15 equiv.) in 1,2-dichloroethane (DCE) [11]. Coincidentally,the Kantam group developed a similar catalysis system for the synthesis of amides from carboxylic acids and DMF. The catalysis system consists of CuO (10 mol%),TBHP (3 equiv.),and DMF (1.5 mL) [12]. All of these works require the use of an excessive amount of DMF,either 15 equiv. or as the solvent. Actually,this drawback is found in many reactions involving DMF as the reactants,which is not only against atom-economic strategy,but also restricts the reactions to those volatile formamides. In addition,the similar amidation of a-oxocarboxylic acids with formamides have been reported [13]. Although the reactions yielded the amiation products in good yield in the presence of only 5 equiv. of formamides in Wang’s work [13a],10 mol% copper catalyst was used. In our work presented here,only 4 equiv. of DMF and 1 mol% copper catalyst are enough to achieve high yields. 2. Experimental
1H NMR and 13C NMR spectra were recorded on Bruker ARX400. High resolution mass spectra were measured on Bruker MicroTOF II ESI-TOF mass spectrometer. All solvents were distilled prior to use unless otherwise noted. 1H NMR spectra were recorded in CDCl3 and referenced to residual CHCl3 at 7.26 ppm,and 13C NMR spectra were referenced to the central peak of CDCl3 at 77.0 ppm.
General procedure for the amidation of benzoic acids: A 50 mL sealed tube (with a Teflon high pressure valve) equipped with a magnetic stir bar was charged with Cu(OTf)2 (0.05 mmol), followed by carboxylic acid (0.5 mmol),formamide (2.0 mmol), tert-butyl peroxide (DTBP,1 mmol),and DCE (1 mL). After the reaction mixture was stirred at 130 8C for 12 h,it was allowed to cool to ambient temperature. The reaction mixture was diluted with ethyl acetate,and then filtered through a small pad of Celite. The filtrate was washed with saturated aqueous NaHCO3 (5 mL) and brine (5 mL,twice). The organic phase was dried (Na2SO4) and concentrated in vacuo. The residue was purified by silica gel preparative TLC to give the corresponding product. 3. Results and discussion
Our research started with the investigation of the amidation of 4-chlorobenzoic acid. In the presence of CuI (10 mol%) as the catalyst and K2S2O8 (2.0 equiv.) as the oxidant,the reaction generated desired product 1 in a very low yield in DMF at 130 8C (Table 1,entry 1). Screening oxidants showed that DTBP was the comparatively better oxidant than the other peroxides (entry 4), and thus,DTBP was chosen as the oxidant for further studies. Among the surveyed copper catalysts,Cu(OTf)2 proved to be the most efficient,and the yield reached 89% (entry 8). It should be noted that even Cu could catalyze the amidation reaction quite effectively (entry 5). In all of the above reactions,DMF was used as the solvent. To circumvent this problem,we turned our attention to reduce the quantities of DMF. To achieve this end,an appropriate solvent should be found. Therefore,we surveyed different solvents with 10 equiv. DMF. While polar solvents including dimethyl acetamide (DMA),DMSO,CH3CN,and 1,4- dioxane gave the amidation product in poor yields,fortunately,an excellent yield was obtained when the reaction was carried out in DCE (entry 13). Gratifyingly,the yield remained excellent even when the amount of DMF was reduced to 4 equiv. (entry 14). The use of 3 equiv. DMF resulted in the decrease of the yield. Notably, only 1% of Cu(OTf)2 was enough to catalyze the reaction efficiently and the yield almost remained unchanged (entry 17). The reaction did not take place in the absence of Cu(OTf)2 (entry 18).
| Table 1 Condition optimization for the amidation of 4-chlorobenzoic acid with DMF. |
With the optimized condition in hand,we started to explore the scope of carboxylic acids. As shown in Table 2,various aromatic carboxylic acids reacted efficiently with DMF to produce the corresponding amides. Benzoic acid and its derivatives bearing an electron-donating group including methyl and methoxyl at ortho-, meta- and para-position were smoothly transformed into corresponding products in good yields (2-8). Notably,halides,including fluoride,chloride,bromide,and even iodide,which are usually reactive in the presence of transition metals such as copper,were compatible with the reaction conditions and all gave excellent results (9-17). Next,we examined the compatibility of benzoic acids containing electron-withdrawing functionalities. A range of electron-withdrawing substituents on aromatic rings like cyano, trifluoromethyl,carbonyl,and ester were well-tolerated (18-25). The presence of nitro groups resulted in diminished yields. Gratifyingly,by increasing the quantity of catalyst Cu(OTf)2 to 5 mol%,the reactions provided the desired products in modest yields (26,27). The amides with acetoxy or phenyl groups could also be prepared from corresponding aromatic carboxylic acids (28,29). In addition,a series of disubstituted aromatic carboxylic acids and 1- or 2-naphthoic acid were reactive to give desired products in moderate to good yields (30-35). Finally aliphatic carboxylic acids could be amidated effectively under the identical conditions (36).
| Table 2 Amidation of benzoic acid and its derivatives with DMF. |
The use of 4 equiv. DMF enables the amidation protocol to be extended to other formamides,especially non-volatile ones. Taking advantage of the strength of this protocol,we examined other formamides with benzoic acids under the identical conditions. As shown in Table 3,the formamides derived from diethylamine and morpholine reacted with benzoic acids to generate the corresponding amides in moderate yields (37,38). Unfortunately,N-methyl-N-phenylformamide was not reactive (39).
| Table 3 Amidation of benzoic acid with different formamides. |
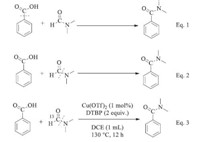
Mechanistically,the carbonyl group of the amide product could be from the carboxyl group of benzoic acids or the amide group of formamides (Fig. 2,Eqs. (1) and (2)). To elucidate the source of the carbonyl group,we carried out a 13C-isotope labeling experiment. Therefore,benzoic acid was allowed to react with 13C-labeled DMF under the standard conditions (Fig. 2,Eq. (3)). MS analysis showed that the resulting N,N-dimethylbenzamide did not contain 13C, which demonstrated that the carbonyl group should come from the carboxyl group of benzoic acid,rather than the amide group of formamides.
|
Download:
|
| Fig. 2. Investigation into the source of the carbonyl groups of the amidation products. | |
In summary,we disclosed a highly efficient approach to form amide bonds from formamides and carboxylic acids. In this protocol,only 1 mol% cheap and relatively less toxic copper catalyst and 4 equiv. formamides were enough to facilitate the reaction. Both aromatic and aliphatic carboxylic acids can be transformed into corresponding amide products with high yields under identical conditions. This reaction showed a broad substrate scope and a wide variety of functional groups were compatible.
AcknowledgmentsThe work was supported by the National Natural Science Foundation of China (No. 21372176), Tongji University 985 Phase III funds, Pujiang Project of Shanghai Science and Technology Commission (No. 11 J1409800), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
We also thank Professor Xiaobing Wan in College of Chemistry, Chemical Engineering and Material Science of Soochow University for the generous donation of 13C-labeled DMF.
| [1] | J.M. Humphrey, A.R. Chamberlin, Chemical synthesis of natural product peptides: coupling methods for the incorporation of noncoded amino, Chem. Rev. 97 (1997) 2243-2266. |
| [2] | (a) V.R. Pattabiraman, J.W. Bode, Rethinking amide bond synthesis, Nature 480 (2011) 471-479;(b) T. Cupido, J. Tulla-Puche, J. Spengler, F. Albericio, The synthesis of naturally occurring peptides and their analogs, Curr. Opin. Drug Discov. Dev. 10 (2007) 768-783;(c) T.J. Deming, Synthetic polypeptides for biomedical applications, Prog. Polym. Sci. 32 (2007) 858-875;(d) H. Lundberg, F. Tinnis, N. Selander, H. Adolfsson, Catalytic amide formation from nonactivated carboxylic acids and amines, Chem. Soc. Rev. 43 (2014) 2714-2742. |
| [3] | (a) S.Y. Han, Y.A. Kim, Recent development of peptide coupling reagents in organic synthesis, Tetrahedron 60 (2004) 2447-2467;(b) E. Valeur, M. Bradley, Amide bond formation: beyond the myth of coupling reagents, Chem. Soc. Rev. 38 (2009) 606-631;(c) A. El-Faham, F. Albericio, Peptide coupling reagents, more than a letter soup, Chem. Rev. 111 (2011) 6557-6602. |
| [4] | C.L. Allen, J.M.J. Williams, Metal-catalysed approaches to amide bond formation, Chem. Soc. Rev. 40 (2011) 3405-3415. |
| [5] | D.J.C. Constable, P.J. Dunn, J.D. Hayler, et al., Key green chemistry research areas - a perspective from pharmaceutical manufacturers, Green Chem. 9 (2007) 411-420. |
| [6] | R.M. Lanigan, T.D. Sheppard, Recent developments in amide synthesis: direct amidation of carboxylic acids and transamidation reactions, Eur. J. Org. Chem. 33 (2013) 7453-7465. |
| [7] | J. Muzart, N,N-Dimethylformamide: much more than a solvent, Tetrahedron 65 (2009) 8313-8323. |
| [8] | S. Ding, N. Jiao, N,N-Dimethylformamide: a multipurpose building block, Angew. Chem. Int. Ed. 51 (2012) 9226-9237. |
| [9] |
For the synthesis of amides with formamides, see: (a) Z. Liu, J. Zhang, S. Chen, et al., Cross coupling of acyl and aminyl radicals: direct synthesis of amides catalyzed by Bu |
| [10] | P.S. Kumar, G.S. Kumar, N.V. Reddy, K.R. Reddy, Copper-catalyzed oxidative coupling of carboxylic acids with N,N-dialkylformamides: an approach to the synthesis of amides, Eur. J. Org. Chem. (2013) 1218-1222. |
| [11] | Y.X. Xie, R.J. Song, X.H. Yang, J.N. Xiang, J.H. Li, Copper-catalyzed amidation of acids using formamides as the amine source, Eur. J. Org. Chem. (2013) 5737-5742. |
| [12] | S. Priyadarshini, P.J. Amal Joseph, M.L. Kantam, Copper catalyzed cross-coupling reactions of carboxylic acids: an expedient route to amides, 5-substituted glactams and a-acyloxy esters, RSC Adv. 3 (2013) 18283-18287. |
| [13] | (a) D. Li, M. Wang, J. Liu, J.Q. Zhao, L. Wang, Cu(Ⅱ)-catalyzed decarboxylative acylation of acyl C-H of formamides with a-oxocarboxylic acids leading to a-ketoamides, Chem. Commun. 49 (2013) 3640-3642;(b) H. Wang, L.N. Guo, X.H. Duan, Copper-catalyzed oxidative condensation of a-oxocarboxylic acids with formamides: synthesis of a-ketoamides, Org. Biomol. Chem. 11 (2013) 4573-4576. |