Ion-selective electrodes (ISEs) as potentiometric sensors are considered useful analytical devices and provide some benefits such as a portable,rapid,inexpensive,and simple method for the determination of ionic species. The selective detection schemes for many anions have the vital drawback of the classical Hofmeister series that is correlated with a preference for hydrophobic anions. Therefore,there is a need for ionophores with improved selectivities and sensitivities in the field of anion-selective electrodes. A very interesting development in the field of ionselective electrodes (ISEs) is the preparation of electrodes that can chemically recognize specific anions and offer potentiometric responses that differ from the conventional anion-exchange based membranes. One of the new carriers with anti-Hofmeister behavior that can be used in the development of potentiometrically chemical sensors should be metal-ligand interactions. Metalloporphyrins [1, 2, 3],metallophthalocyanines [4],cyclometallic amines [5],Schiff base complexes [6, 7, 8],and N-4-metal complexes [9, 10, 11] have been observed to show such specific metal-ligand interactions. ISEs based on these metal-ligand interactions have been reported for the determination of thiocyanate anion,and they showed deviation from the classical Hofmeister series. The thiocyanate ion is usually present in low concentrations in human serum,saliva,and urine as a result of the digestion of some vegetables of the genusBrassicacontaining glucosinolates (cabbage,turnip and kale) or by intake of thiocyanate-containing foods such as milk and cheese. Higher concentration of this ion,which is a metabolic product of cyanide, arises from tobacco smoke [12]. In this respect,the concentration level of thiocyanate is considered to be a good probe to distinguish between smokers and non-smokers. It has been found that there is a statistical correlation between blood cyanide,plasma thiocyanate,and salivary thiocyanate [13]. Several thiocyanate ISEs based on the variety of ion carriers have also been reported in Refs. [6, 8, 14, 15, 16, 17, 18, 19, 20, 21]. Some sensors have narrow linear range [18],narrow effective pH range [6, 21],and high detection limit [18],and some suffer serious interference from anions like CN- [8, 16],Cl- [18] and SO42- [16]. In this work we constructed a PVC membrane electrode based on bis(N-3-methylphenyl salicylidenaminato)copper(II) as ionophore,and the interaction between the ionophoreandthiocyanateionwas investigated using UV-vis absorption spectroscopy. The effect of membrane composition,pH,and internal solution on the electrode response was investigated. This electrode is appropriate for measuring the concentration of thiocyanate in different samples without pretreatment step and without important interferences from other anionic species. This electrode is easy to fabricate,shows high selectivity and sensitivity,wide dynamic range,low detection limit,and fast response time. 2. Experimental
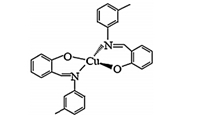
Reagents used in this study are as follows: Di-butyl phthalate softeners (DBP),cationic salt hexadecyl trimethyl ammonium bromide (HTAB),the solvent tetrahydrofuran (THF),polyvinyl chloride (PVC),sodium or potassium salts of the anions,and nitric acid (all from Merck) were of the highest purity available and used without further purification. The ionophore used for manufacturing the membrane is bis(N-3-methylphenyl salicylidenaminato)-copper(II) [(Cu(MSA)2)],which is shown in Scheme 1. The Cu(MSA)2 was synthesized by using the reported procedure [22].
|
Download:
|
| Scheme 1. Structure of ionophore used in the manufacture of thiocyanate ion selective electrode. | |
The potential and pH measurements were carried out on a pH/mV meter (Metrohm model 691). The PVC membrane electrode and Ag/AgCl/KCl 3.0 mol/L were used as the indicator and reference, respectively. All measurements were performed using this cell diagram:
Ag|AgCl,KCl (3 mol/L)jinternal solution,KSCN (1.0×10-3 mol/L)|membrane|test solutionjKCl (3 mol/L)|AgCl|Ag.
The general method for the preparation of PVC membrane electrode was as follows: 31 mg PVC,61 mg DBP,5 mg ionophore Cu(MSA)2 and 3 mg HTAB cationic additive were mixed and dissolved in 3 mL THF. The mixture was poured into a crystallizer with 2 cm diameter and allowed to slowly evaporate to a thick oil mixture. Then a plastic conical tube 10 cm in length and 3 mm in diameter was immersed in the oil mixture for 10 s until a thin layer with thickness of 0.3 mm was formed. The tube was pulled out from the mixture and kept at room temperature for 12 h to dry. Then,it was filled with an internal solution of 1.0×10-3 mol/L KSCN. The electrode was finally conditioned for 12 h by soaking in 1.0×10-2 mol/L potassium thiocyanate. A silver-silver chloride electrode was used as an internal reference electrode. 3. Results and discussion
To determine which anion the electrode is most sensitive to,a large range of anions were examined in the concentration range of 1×10-5 -1×10-1 mol/L. Ligand (N-3-methylphenyl salicylidenaminato),due to the nitrogen and oxygen atoms in its structure, appears to be a neutral factor in preparing a PVC membrane electrode for anions. The membrane showed the best selectivity for thiocyanate ion among the most common inorganic anions.
To verify the electrode’s selectivity for the thiocyanate ion,the interaction between the ionophore and thiocyanate ion was investigated using UV-vis absorption spectroscopy [14]. For this purpose,the UV-vis absorption spectrum of 5.0×10-5 mol/L ionophore alone and then a solution of 5.0×10-5 mol/L ionophore and thiocyanate were taken in THF solvent. The resulting spectra showed a significant interaction with the ionophore and thiocyanate ions. Specific interactions between the central metal and the thiocyanate ions can be seen clearly. The maximum intensity of the absorption spectrum,which corresponds to 393 nm in ionophore alone,in the presence of thiocyanate ions is transferred to 332 nm. It may be due to the unique interactions between the central metal copper(II) and thiocyanate ion in (Cu(MSA)2). 3.1. The effect of membrane composition,pH and internal solution on the electrode response
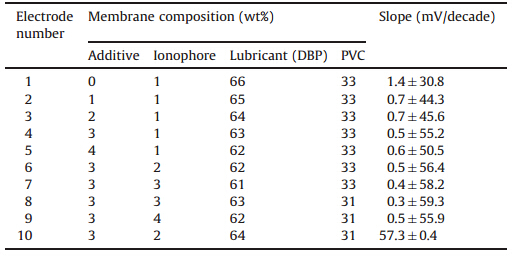
Considering some of the important characteristics of PVC membranes such as the nature and amount of ionophore and softeners,ratio of softener to PVC,and effect of the additives on the sensitivity and selectivity of the membrane [23],the effects of membrane composition on the potential response of the electrode were examined. In this series of experiments,the ionophore and additives in the membranes were changed and the optimal composition and conditions for the electrode were determined by using calibration curves (Table 1). Then,the effect of ionophore and additives were investigated. To determine the optimal amount of the cationic additive,the amount of additive was increased from 0% to 4% while the amount of ionophore and PVC were held constant and DBP was reduced. According to the table the best slope was obtained when the additive amount was equal to 3%. Next,the ionophore was changed from 1% to 4% while the additive was held at the optimal 3% and DBP and PVC were adjusted as specified The best response was achieved at membrane No. 8,which was composed of ionophore (Cu(MSA)2):HTAB:DBP:PVC with relative percentage of 3:3:63:31.
| Table 1 Optimization of membrane composition for thiocyanate selective electrode based on ionophore Cu(MSA)2. |
The effect of pH on the electrode response was investigated using 1.0×10-3 mol/L potassium thiocyanate at different pHs. Nitric acid and sodium hydroxide were used to change the pH of the test solution. Potential response was plotted against the pH of solution. Studies indicate the electrode response is independent of the pH test solution in the range of pH 4.0-10.0. Potential changes in pH less than 4.0 presumably are due to the instability of the ionophore due to protonation of nitrogen. Also,at pH higher than 10.0 intensive changes in potential likely are due to the sensor response to hydroxide ions.
Concentration of potassium thiocyanate as the internal solution of the electrode was changed from 1.0×10-4 mol/L to 1.0×10-2 mol/L,and the potential of response electrode was read. It was found that the changes in the concentration of internal solution have no significant effect on the potential slope; only the intercept of curves changes,so the solution of 1.0×10-3 mol/L potassium thiocyanate was used as an internal solution in the measurements. 3.2. Response time,dynamic range,reproducibility and selectivity
Response time was tested by measuring the time required to get a steady state potential (±1 mV) after successive additions of thiocyanate ion. To determine the response time of the electrode,the potentials of solutions with different concentrations of thiocyanate ions at regular intervals were obtained from 1.0×10-6 mol/L to 1.0×10-1 mol/L by emf measurements. The static response times of different electrodes were below 21 s (9-21 s). Usually,ion selective electrodes have the advantage that they have a very short response time.
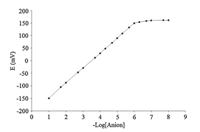
After optimization of the variables affecting the response of the electrode,the electrode with optimal membrane composition (No. 8) was used for measuring potential in the concentration range of 1.0×10-8 -1.0×10-1 mol/L. As seen in Fig. 1,the electrode response to thiocyanate ion is linear in the range of 1.0×10-6 -1.0×10-1 mol/L with a slope of 59.3±0.3 mV/decade. The detection limit was obtained to be 5.0×10-7 mol/L by extrapolation.
|
Download:
|
| Fig. 1. Calibration curve of ion selective thiocyanate electrode based on ionophore Cu(MSA)2. | |
To determine the reproducibility of the electrode results the standard deviation of electrode response for 1.0×10-2 mol/L,1.0×10-3 mol/L,and 1.0×10-4 mol/L of potassium thiocyanate was measured in half hour intervals. The standard deviation of the electrode response was obtained to be±0.3,±0.4,and±0.8 mV, respectively,over 10 measurements,which shows good repeatability of the electrode. Various factors,such as leakages of the softener, ionophore,or additive reduce the electrode’s lifespan. To reduce the leakage of the membrane components,it can be stored in distilled water or dry. These studies were carried out,and the results show that after 70 days the electrode slope and the linear range decreased to 55.4±0.6 mV/decade and 2.0×10-5 -1.0×10-1 mol/L,respectively.
Perhaps the most important feature of an ion selective electrode is selectivity,defined by response of an electrode to a specific ion in the presence of other ions. In this work,the selectivity coefficients were obtained by fixed interference method (FIM). The electromotive force (emf) of the cell was measured for solutions of constant activity of the interfering ion,aB(2.0×10-1 mol/L),and varying activity of the main ion,aA(2.0×10-7 -2.0×10-1 mol/L potassium thiocyanate). The emf values obtained were plottedvs. the logarithm of the activity of the main ion. The intersection of the extrapolated linear portions of this plot indicates the value ofaA that is to be used to calculateKA,Baccording to Eq. (1)

Response reflects the fact that the interfering ions do not confuse ion-selective membrane performance considerably. According to the Hofmeister series (ClO4- >SCN- >I- >NO3- >Br- >NO2- > Cl- >SO42- ),the anti Hofmeister sequence can be observed for ions: I- =-1.9>NO2- =-2.7>NO3- =Br- =ClO3- =-2.8> CN- =-3.0>Cl- =CrO42- =-3.3>F-=-3.5>IO3- =-3.6>SO32- =BrO3-=SO42-=-3.7>CO32- =-3.8.
The observed deviation from the Hofmeister series for high lipophilic anion is due to the interaction of the anion with the central metallic core ionophore. Selectivity coefficients show that the prepared selective electrode is highly selective for the thiocyanate ion,and only the iodide ion is an almost serious interferential anion.
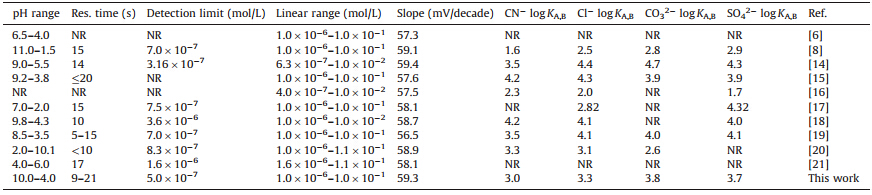
The performance characteristics of the proposed electrode are compared with those of the thiocyanate PVC membrane electrodes previously reported in Table 2. The slope of the proposed electrode is more Nerstian than other previously reported electrodes. The working pH range of the proposed electrode is wider than other reported electrodes (except Ref. [8]) and the detection limit of the electrode is also lower than others except Ref. [14].
| Table 2 Comparison of prepared thiocyanate ion selective electrode with the similar electrodes (NR = not reported). |
The membrane electrode was used as an indicator electrode for determining the endpoint in potentiometric titration. 20 mL of 1.0×10-3 mol/L potassium thiocyanate solution was titrated with 1.0×10-2 mol/L solution of silver nitrate by the potentiometric method. The slope of the titration curve at the equivalence point is relatively sharp. To determine the end point volume more exactly,the first derivative plot (ΔE/ΔV versus V) was used. The exact thiocyanate concentration can be determined this way.
In order to test the analytical utility of the sensor,samples of saliva and urine of a smoker and a non smoker were analyzed. The samples were diluted in a 1:10 ratio using phosphoric acid and potassium hydroxide to set the pH 5.0. The concentration of thiocyanate was determined by the proposed method and compared with its concentration as determined by standard spectrophotometric method [24]. The mean and standard deviation over four trials measured by the proposed and standard methods are compared in Table 3. The comparison of the obtained amounts in each sample using two methods in 95% confidence level confirms that no significant difference is observed.
| Table 3 Comparison of proposed method and spectrophotometric standard method for determination of thiocyanate in different samples (thetvalue was used at 95% confidence level[25]). |
A highly selective electrode is described for thiocyanate ion based on PVC membrane electrode of bis(N-3-methylphenyl salicylidenaminato)copper(II) as ionophore. This electrode is easy to prepare,shows high selectivity and sensitivity,wide dynamic range,low detection limit,and fast response time. These properties make this electrode suitable for measuring the concentration of thiocyanate in a wide variety of samples without the need for pretreatment steps and without significant interference from other anionic species present in the sample.
| [1] | Y. Xie, F. Zhang, L. Pingle, F. Hao, H. Luo, Catalytic oxidation of cyclohexane with dioxygen over boehmite supported trans-A2B2 type metalloporphyrins catalyst, J. Mol. Catal. 386 (2014) 95-100. |
| [2] | W.J. Sun, J. Li, G.P. Yao, F.X. Zhang, J.L. Wang, Surface-modification of TiO2 with new metalloporphyrins and their photocatalytic activity in the degradation of 4-notrophenol, J. Appl. Surf. Sci. 258 (2011) 940-945. |
| [3] | M. Penza, R. Rossi, M. Alvisi, M.A. Signore, Metalloporphyrins modified carbon nanotubes networked films-based chemical sensors for enhanced gas sensitivity, Sens. Actuators B 144 (2010) 387-394. |
| [4] | D. Arıcan, M. Arıcı, A. Lü tfi Uğur, A. Erdoğmuş, Effects of peripheral and nonperipheral substitution to the spectroscopic, electrochemical and spectroelectrochemical properties of metallophthalocyanines, Electrochim. Acta 106 (2013) 541-555. |
| [5] | S. Pedreno, C. Ortuno, J.A. Martinez, Anion selective polymeric membrane electrodes based on cyclopalladated amine complexes, Talanta 47 (1998) 305-310. |
| [6] | Z.Q. Li, Z.Y. Wu, R. Yuan, et al., Thiocyanate-selective PVC membrane electrodes based on Mn(Ⅱ) complex of N,N'-bis-(4-phenylazosalicylidene) o-phenylene diamine as a neutral carrier, J. Electrochim. Acta 44 (1999) 2543-2548. |
| [7] | Z.Y. Sun, R. Yuan, Y.Q. Chai, et al., Study of a bis-furaldehyde Schiff base copper(Ⅱ) complex as carrier for preparation of highly selective thiocyanate electrodes, Anal. Bioanal. Chem. 378 (2004) 490-494. |
| [8] | M.R. Ganjali, T. Poursaberi, F. Basiripour, et al., Highly selective thiocyanate poly(vinyl chloride) membrane electrode based on a Cadmium-Schiff's base complex, J. Anal. Chem. 370 (2001) 1091-1095. |
| [9] | A. Abbaspour, M.A. Kamyabi, A.R. Esmaeilbeig, R. Kia, Thiocyanate selective electrode based on unsymmetrical benzoN4 nickel(Ⅱ) macrocyclic complexes, Talanta 57 (2002) 859-867. |
| [10] | M.M. Ardakani, A.A. Ensafi, M.S. Niasari, S.M. Chahooki, Selective thiocyanate poly(vinyl chloride) membrane based on a 1,8-dibenzyl-1,3,6,8,10,13-hexaazacyclotetradecane-Ni(Ⅱ) perchlorate, Anal. Chim. Acta 462 (2002) 25-30. |
| [11] | T. Poursaberi, M. Salavati Niassari, S. Khodabakhsh, et al., A selective membrane electrode for thiocyanate ion based on a copper-1,8-dimethyl-1,3,6,8,10,13-hexaazayclotetradecane complex as ionophore, Anal. Lett. 34 (2001) 2621-2632. |
| [12] | R.E. Bliss, K.A. O'Connell, Problems with thiocyanate as an index of smoking status: a critical review with suggestions with improving the usefulness of biochemical measures in smoking cessation research, Health Psychol. 3 (1984) 563-581. |
| [13] | B. Tossanaitada, T. Masadome, T. Imato, Sequential injection analysis of thiocyanate ions using a microfluidic polymer chip with an embedded ion-selective electrode, Anal. Sci. 30 (2014) 507-511. |
| [14] | A.K. Singh, U.P. Singh, S. Mehtab, Thiocyanate selective sensor based on tripodal zinc complex for direct determination of thiocyanate in biological samples, Sens. Actuators B 125 (2007) 453-461. |
| [15] | M. Aravand, M.A. Zanjanchi, L. Heydari, Novel thiocyanate-selective membrane sensor based on crown ether-cetyltrimethyl ammonium thiocyanate ion-pair as a suitable ionophore, Sens. Actuators B 122 (2007) 301-308. |
| [16] | P. Buhlmann, L. Yahya, R. Endress, Ion-selective electrodes for thiocyanate based on the dinuclear zinc(Ⅱ) complex of a bis-N,O-bidentate Schiff base, Electroanalysis 16 (2004) 973-978. |
| [17] | W.J. Xu, Y.Q. Chai, R. Yuan, S.L. Liu, A novel thiocyanate-selective electrode based on a zinc-phthalocyanine complex, Anal. Bioanal. Chem. 385 (2006) 926-930. |
| [18] | M.J. Segui, J. Lizondo Sabater, R. Martinez Manez, Linear polyamines as carriers in thiocyanate-selective membrane electrodes, Talanta 68 (2005) 1182-1189. |
| [19] | M.M. Ardakani, A. Sadeghi, M. Salavati Niasari, Highly selective thiocyanate membrane electrode based on butane-2,3-dione bis(salicylhydrazonato)zinc(Ⅱ) complex, Talanta 66 (2005) 837-843. |
| [20] | A. Badri, P. Pouladsaz, Highly selective and sensitive thiocyanate PVC membrane electrodes based on modified Zeolite ZSM-5, Int. J. Electrochem. Sci. 6 (2011) 3178-3195. |
| [21] | W.S. Han, T.K. Hong, Y.H. Lee, Thiocyanate ion selective solid contact electrode based on Mn complex of N,N'-bis-(4-phenylazosalicylidene)-O-phenylene diamine ionophore, J. Am. Anal. Chem. 2 (2011) 731-738. |
| [22] | R. Vafazadeh, V. Hayeri, A.C. Willis, Synthesis, crystal structure and electronic properties of bis(N-2-bromophenyl-salicydenaminato)copper(Ⅱ) complex, Polyhedron 29 (2010) 1810-1814. |
| [23] | E. Bakker, P. Buehlman, E. Pretsch, Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics, Chem. Rev. 97 (1997) 3083-3132. |
| [24] | G.W. Whiston, The determination of thiocyanate in coal-carbonising plant effluents, sewage works influents and effluents and polluted waters, Analyst 87 (1962) 819-823. |
| [25] | J.C. Miller, J.N. Miller, Statistics for Analytical Chemistry, 2nd ed., Ellis Horwood, Chichester, 1988. |