b. Key Laboratory of Marine New Materials and Related Technology, Zhejiang Key Laboratory of Marine Materials and Protection Technology, Ningbo Institute of Material Technology & Engineering, Chinese Academy of Sciences, Ningbo 315201, China;
c. Ningbo University of Technology, Ningbo 315211, China
Tissue engineering,which combines a biodegradable scaffold, living cells and/or biologically active molecules,is a promising approach to promote the repair and regeneration of targeted tissues [1]. For a scaffold to be used in hard tissue reconstruction,it must meet certain requirements such as high porosity,biocompatibility, biodegradability and sufficient mechanical strength [2, 3]. Several natural and synthetic polymers have been used to fabricate the scaffolds,such as collagen [4],silk fibroin [5],poly(lactic acid) (PLA) [6],poly(caprolactone) (PCL) [7],poly(lactic-co-glycolic) (PLGA) [8] among others [9]. Microbially produced polyhydroxyalkanoates (PHAs),which are fully biodegradable polyesters,have been considered as promising biomaterials for the applications in both conventional medical devices and tissue engineering because of their good biocompatibility,broad range of mechanical properties and easy processability [10]. Compared to poly(3-hydroxybutyrate) (PHB),which is the most widely used PHAs in tissue engineering; PHBV has lower stiffness and brittleness,higher flexibility and toughness [11]. Beside these unique mechanical properties,PHBV also shows good biocompatibility. Köse et al. synthesized macroporous PHBVmatrices by the porogen leachingmethod,and studied the proliferation of rat osteoblasts on the PHBV matrices. Results showed that osteoblasts could grow inside the PHBV matrices and lead to mineralization,which suggest that PHBV is a promising polymer matrix material for bone tissue engineering [12].
Chitin is known for its unique biochemical characteristics in the area of wound healing and skin regeneration [13]. Recently,chitin and its derivatives chitosan were reported to promote osteogenic cell recruitment and attachment thus facilitate bone formation [14]. Chitin crystallites in nanoscale can be obtained by acidolysis the amorphous domains of chitin [15]. Chitin nanocrystals have crystalline microfibrillar structure and possess high stiffness and strength,and they have been widely used as effective reinforcing filler for many types of biomaterial matrices such as poly(vinyl alcohol) (PVA) [16],PCL [17],chitosan [18],alginate [19] among others [20]. Chitin nanocrystals composites have great potential as a scaffold in tissue engineering.Wongpanit et al. have incorporated the chitin nanocrystals as nanofiller into silkfibroin sponge. The presence of chitin nanocrystals not only improved the scaffold dimensional stability and compression strength but also promoted mouse fibroblast cells spreading [21]. Hariraksapitak et al. have prepared a chitinwhisker reinforced hyaluronan-gelatin scaffold andfoundthe incorporation of chitin whisker increased scaffold tension strength.The composite scaffold with 10% chitin whisker supported the proliferation of human osteosarcoma cells [22].
Human adipose-derived stem cells (hADSCs) have the potential to undergo osteogenesis [23],chondrogenesis [24],adipogenesis and other processes [25]. Besides their high osteogenic potential, hADSCs can be easily obtained with large quantities and low donor site morbidity,which render them ideal seed cells in tissue engineering [23]. In this research,a porous PHBV/CNC composite scaffold is synthesized by the TIPS and salts leaching method. The biocompatibility of this composite scaffold is investigated by in vitro hADSCs culture. The growth of hADSCs on the PHBV/CNC composite scaffold was studied. 2. Experimental 2.1. Materials
PHBV containing 20% molar hydroxyvalerate (HV) was supplied by Tianan Biologic Co.,Ltd. (Ningbo,China). The average molecular weight (Mw) was approximately 250,000 g/mol. 1,4-Dioxane and sodium chloridewere purchased fromSinopharm Chemical Reagent Co.,Ltd. (Shanghai,China). Chitin from shrimp shells was purchased from Aladdin reagent Co.,Ltd. (Shanghai,China) and chitin nanocrystals (CNC) were prepared through acid hydrolysis as described in our previous report [26]. hADSCs (human adiposederived stem cells),hADSC-GM (human adipose-derived stem cell growthmedium) were purchased fromBiowit Technologies (Shenzhen, China),trypsin-EDTA was purchased from Gibco Invitrogen. 2.2. Preparation of the PHBV scaffold and PHBV/CNC composite scaffold
The PHBV scaffolds were fabricated using the thermally induced phase separation and salts leaching technique. Firstly,PHBV was dissolved in dioxane (10%,wt/v) and the solution was stirred at 100 ℃ for 2 h with N2 as the protecting gas to obtain a homogeneous solution. Then the sieved sodium chloride (100-300 µm) particles were added into the PHBV solution and mixed by vortex for 5 min. The mass ratio of the salt particles to PHBVwas 1:1. Then the mixed solution was casted into a pre-warmed mold and cooled to -18 ℃. After incubated at -18 ℃ for 2 h,the sampleswere put into distilled water at roomtemperature to exchange solvent andsodiumchloride for 2 days. Then the obtained porous scaffoldswere dried at 25 ℃ for 2 days. For the synthesis of the PHBV/CNC composite scaffold,a PHBV solution was produced by dissolving PHBV in dioxane as previously mentioned,then CNC (10 wt% of PHBV) were ultrasonically dispersed in dioxane for 30min and added into the PHBV solution. The PHBV/CNC mixed solution was stirred for 30 min and then sieved sodium chloride (100-300 µm) particles were added into the solution. Phase separation and solvent exchange were processed in a same manner as those described above. 2.3. Characterization of PHBV/CNC composite scaffolds
Transmission electron micrograph (TEM) measurements (FEI Tecnai G2 F20,accelerating voltage 200 kV) were carried out to observe the morphology of the CNC. The CNC were dispersed in a 0.5% ethanol solution. Samples were prepared by evaporating a drop of this solution on a carbon coated copper grid. Scanning electron microscopy (SEM) images of the scaffolds were observed (Hitachi TM-1000). Cross-sections of the scaffolds were frozen fractured and coated with gold before examination. The surface chemistry of the scaffolds was studied using X-ray photoelectron spectroscopy (XPS) (AXIS UTLTRADLD). The hydrophilicity of the scaffold was evaluated through a static contact angle system (Dataphysics OCA). The scaffold was compressed to form a plane surface and approximately 2 µL of water was dropped on the surface. Three samples were tested for each scaffold. The mechanical properties of the scaffolds were analyzed by a compression test using an INSTRON universal testing machine. The scaffolds were cut into circular specimens of 12 mm in diameter and 5 mmin thickness. The crosshead speed was 0.5 mm/ min. The compressive modulus was defined as the slope of the initial linear portion of the stress-strain curve. At least five specimens were tested for each scaffold. 2.4. Cell attachment
hADSCswere cultured inHADSC-GMsupplemented with 5% FBS, 1% antibiotics (100 units/mL penicillin,100 units/mL streptomycin) and 1% MSCGS (Mesenchymal Stem Cell Growth Supplement). The cultures were maintained at 37 ℃ in a humidified atmosphere containing 5% CO2. The scaffoldswere cut into cylindrical specimens of 12 mm × 5 mm and were stuck to the culture plate bottomusing Silastic® (Medical grade) to avoid floating. Then the scaffolds were sterilized with 75% ethanol (2 h),washed with a phosphate buffer solution (PBS) three times. hADSCs were trypsinized and counted,and then seeded (7 × 105 cells/mL) on the PHBV and PHBV/CNC scaffolds in 24-well plates. The culture mediumwas changed every two days. Cell attachment and distribution on the scaffold were investigated using a confocal laser scanning microscope (Leica TCS SP5) after 7 days of incubation. The unattached cells were rinsed by PBS and then removed. The cell/scaffold constructs were stained by DAPI and fluorescent images were taken. 3. Results and discussion 3.1. Morphologies of chitin nanocrystals and PHBV/CNC scaffold
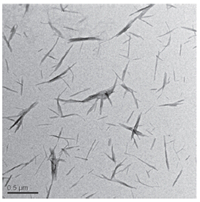
Fig. 1 shows the morphology of chitin nanocrystals after acid hydrolysis. The length of chitin nanocrystals is 200-500 nm and the width is 20-40 nm. The size and shape of nanoparticles have a critical effect on the scaffold mechanical property. Roohani- Esfahani et al. examined three different shapes (needle,spherical, rod-like) of HA nanoparticles,and they demonstrated that the needle shaped (average width of 25 nm and length of 110 nm) HAcomposite- coated scaffold displayed the highest compressive strength when compared to the other two [27]. Thus these needle-like chitin nanocrystals could be suitable nanofillers for scaffold reinforcement.
|
Download:
|
| Fig. 1.TEM image of chitin nanocrystals. | |
The morphologies of the PHBV and PHBV/CNC composite scaffold were examined by SEM (Fig. 2). Both scaffolds have multiscale pore structures. The macro-pores in the scaffolds mimic the cubic salt particles and the average diameter of macro-pores is between 100 and 300 µm. The macro-pores show inter-connectivity due to the salt particles distribution,the micro-pores (9.6 ± 1.8 µm) that result from phase separation on the macro-pore wall increase the inter-connectivity. As shown,the pore structures and pore size do not change significantly while the chitin nanocrystals incorporated into the scaffold. The scaffolds-mixed solution was incubated below the melting point of dioxane,thus the solvent underwent crystallization and induced a solid-liquid phase separation. The polymer was expelled from the solvent crystals front [28]. Consequently,the micro-porous scaffold was formed after the solvent exchange. Studies suggest that micro-porous structure (pore size < 10 µm) results in larger surface area and contributes to the higher protein adsorption. The macro-pores can also facilitate nutrient diffusion in the microenvironment and provides attachment points for cell adhesion. In other words,the micro-porous scaffolds we fabricated could benefit the cell growth and proliferation [29, 30].

|
Download:
|
| Fig. 2.SEM image of PHBV scaffold (a,b) and PHBV/CNC composite scaffold (c,d). The scales in (a) and (c) are 500 µm and in (b) and (d) are 100 µm. | |
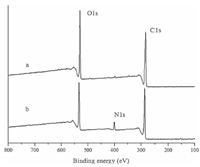
The surface chemistry of PHBV/CNC composite scaffold was studied by XPS. The full XPS spectra of both scaffolds are shown in Fig. 3. The peaks around 286 eV and 532 eV were assigned to the 1s orbital electrons of carbon and oxygen,respectively. The PHBV/ CNC composite scaffold has one more peak around 399 eV,which represented the 1s orbital electrons of nitrogen. Chitin has a - NHCO- function group in its molecular chains,thus the presence of N1s confirmed that chitin nanocrystals were successfully incorporated into the PHBV matrix. The chitin nanocrystals in the PHBV scaffold changes the surface polarity. As a result,the static water contact angle decreases from 88.5 ± 1.5° to 78 ± 1.7°,indicating the improvement of scaffold hydrophilicity. The adhesion and proliferation behavior of cells on polymeric materials depend on the surface characteristics such as wettability,functional groups and roughness [31]. Tamada et al. [32] reported that the polymer surface with awater contact angle of about 70° could provide the most suitable surface for cell adhesion. The addition of CNC into the PHBV matrix may provide more appropriate surface to accommodate cell adhesion,whichwould be surely beneficial to cell proliferation and differentiation.
|
Download:
|
| Fig. 3.XPS specras of PHBV scaffold (a) and PHBV/CNC composite (b) scaffold. | |
The compressive stress-strain curves and compressive modulus of PHBV scaffolds are shown in Fig. 4. At the beginning of the stress-strain curve,the stress increases slowly with increased strain,which indicates that both scaffolds have good flexibility and toughness. The initial liner elasticity around 1% strain is associated with the pore wall bending and the long plateau in the stress- strain curves is related to the collapse of the pore wall. When the strain reached 60%,the compressive stress increased rapidly. Possibly because the pore walls touch each other and form solid bulks under increasing stress [33]. The compressive modulus increases from 5.21 ± 0.14 MPa to 7.12 ± 0.24 MPa with the addition of CNC (the compressive modulus was the slope that calculated from the initial linear portion of stress-strain curves),which indicates that the PHBV/CNC composite scaffolds enhanced mechanical properties as compared to the original PHBV scaffolds. The substantial enhancement of mechanical property may be due to the interactions between the matrix and the chitin nanocrystals through hydrogen bonds [34].

|
Download:
|
| Fig. 4.Compressive stress-strain curves and compressive modulus of PHBV and PHBV/CNC composite scaffold. | |
Cell attachment and distribution on the scaffolds were analyzed by confocal microscopy. In Fig. 5,the cell nuclei were stained blue with DAPI. After 7 days of incubation,more hADSCs attached on and infiltrated into the PHBV/CNC scaffold while the cells seeded on the PHBV scaffold mainly attached on the surface. The incorporation of CNC into the PHBV scaffold may promote bioactivity of the scaffold,thus the cell attachment behavior was improved.

|
Download:
|
| Fig. 5.Confocal microscopy images of hADSCs after 7 days culture on PHBV scaffold (a) and PHBV/CNC scaffold (b). | |
Multi-scale porous PHBV/CNC composite scaffolds were successfully synthesized by a combination of the thermally induced phase separation and salt leaching method. The incorporation of chitin nanocrystals not only changed the scaffold surface chemistry and hydrophilicity,but also improved the mechanical properties of the composite scaffold. Compared to pure PHBV scaffolds,the nano-composite scaffolds had better biocompatibility and promoted the hADSCs adhesion on the scaffold. These results showed that the PHBV/CNC composite scaffolds could be used as potential substrates for tissue engineering.
AcknowledgmentsWegratefully acknowledgeMinistry of Science and Technology of the People’s Republic of China (No. 1106),Ningbo Science Foundation (No. 2011A610116),National Key Basic Research Program of China (973,No. 2014CB643305) and the China Postdoctoral Science Foundation Funded Project (No. 2012T50564) for financial support.
| [1] | R. Cancedda, B. Dozin, P. Giannoni, R. Quarto, Tissue engineering and cell therapy of cartilage and bone, Matrix Biol. 22 (2003) 81-91. |
| [2] | R. Langer, D.A. Tirrell, Designing materials for biology and medicine, Nature 428 (2004) 487-492. |
| [3] | P. Lichte, H.C. Pape, T. Pufe, P. Kobbe, H. Fischer, Scaffolds for bone healing: concepts, materials and evidence, Injury 42 (2011) 569-573. |
| [4] | L.B. Rocha, G. Goissis, M.A. Rossi, Biocompatibility of anionic collagen matrix as scaffold for bone healing, Biomaterials 23 (2002) 449-456. |
| [5] | H.J. Jin, J. Chen, V. Karageorgiou, G.H. Altman, D.L. Kaplan, Human bone marrow stromal cell responses on electrospun silk fibroin mats, Biomaterials 25 (2004) 1039-1047. |
| [6] | F. Zhang, F. Mei, X.Z. Wang, et al., A new route for preparation of β-TCP/PLLA composite, Chin. Chem. Lett. 17 (2006) 883-886. |
| [7] | J.M. Williams, A. Adewunmi, R.M. Schek, et al., Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering, Biomaterials 26 (2005) 4817-4827. |
| [8] | A.S. Goldstein, T.M. Juarez, C.D. Helmke, M.C. Gustin, A.G. Mikos, Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds, Biomaterials 22 (2001) 1279-1288. |
| [9] | D. Puppi, F. Chiellini, A.M. Piras, E. Chiellini, Polymeric materials for bone and cartilage repair, Prog. Polym. Sci. 35 (2010) 403-440. |
| [10] | G.Q. Chen, Q. Wu, The application of polyhydroxyalkanoates as tissue engineering materials, Biomaterials 26 (2005) 6565-6678. |
| [11] | B. Laycock, P. Halley, S. Pratt, A. Werker, P. Lant, The chemomechanical properties of microbial polyhydroxyalkanoates, Prog. Polym. Sci. 38 (2013) 536-583. |
| [12] | G.T. Kö se, F. Korkusuz, P. Korkusuz, et al., Bone generation on PHBV matrices: an in vitro study, Biomaterials 24 (2003) 4999-5007. |
| [13] | R.A.A. Muzzarelli, Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone, Carbohydr. Polym. 76 (2009) 167-182. |
| [14] | Y. Maeda, R. Jayakumar, H. Nagahama, T. Furuike, H. Tamura, Synthesis, characterization and bioactivity studies of novel beta-chitin scaffolds for tissue-engineering applications, Int. J. Biol. Macromol. 42 (2008) 463-477. |
| [15] | J.B. Zeng, Y.S. He, S.L. Li, Y.Z. Wang, Chitin whiskers: an overview, Biomacromolecules 13 (2011) 1-11. |
| [16] | J. Jirawut, R. Ratana, S. Pitt, Fabrication of α-chitin whisker-reinforced poly(vinyl alcohol) nanocomposite nanofibres by electrospinning, Nanotechnology 17 (2006) 4519-4528. |
| [17] | L.D. Feng, Z.Y. Zhou, A. Dufresne, et al., Structure and properties of new thermoforming bionanocomposites based on chitin whisker-graft-polycaprolactone, J. Appl. Polym. Sci. 112 (2009) 2830-2837. |
| [18] | J. Sriupayo, P. Supaphol, J. Blackwell, R. Rujiravanit, Preparation and characterization of α-chitin whisker-reinforced chitosan nanocomposite films with or without heat treatment, Carbohydr. Polym. 62 (2005) 130-136. |
| [19] | A. Watthanaphanit, P. Supaphol, H. Tamura, S. Tokura, R. Rujiravanit, Fabrication, structure, and properties of chitin whisker-reinforced alginate nanocomposite fibers, J, Appl. Polym. Sci. 110 (2008) 890-899. |
| [20] | R. Jayakumar, D. Menon, K. Manzoor, S.V. Nair, H. Tamura, Biomedical applications of chitin and chitosan based nanomaterials -a short review, Carbohydr. Polym. 82 (2010) 227-232. |
| [21] | P. Wongpanit, N. Sanchavanakit, P. Pavasant, et al., Preparation and characterization of chitin whisker-reinforced silk fibroin nanocomposite sponges, Eur. Polym. J. 43 (2007) 4123-4135. |
| [22] | P. Hariraksapitak, P. Supaphol, Preparation and properties of α-chitin-whiskerreinforced hyaluronan-gelatin nanocomposite scaffolds, J. Appl. Polym. Sci. 117 (2010) 3406-3418. |
| [23] | W. Wagner, F. Wein, A. Seckinger, et al., Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood, Exp. Hematol. 33 (2005) 1402-1416. |
| [24] | G.R. Erickson, J.M. Gimble, D.M. Franklin, et al., Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo, Biochem. Biophys. Res. Commun. 290 (2002) 763-769. |
| [25] | J.M. Gimble, F. Guilak, Adipose-derived adult stem cells: isolation, characterization, and differentiation potential, Cytotherapy 5 (2003) 362-369. |
| [26] | B.J. Wang, J. Li, J.Q. Zhang, et al., Thermo-mechanical properties of the composite made of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and acetylated chitin nanocrystals, Carbohydr. Polym. 95 (2013) 100-106. |
| [27] | S.I. Roohani-Esfahani, S. Nouri-Khorasani, Z. Lu, R. Appleyard, H. Zreiqat, The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite-PCL composites, Biomaterials 31 (2010) 5498-5509. |
| [28] | G. Wei, P.X. Ma, Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering, Biomaterials 25 (2004) 4749-4757. |
| [29] | V. Karageorgiou, D. Kaplan, Porosity of 3D biomaterial scaffolds and osteogenesis, Biomaterials 26 (2005) 5474-5491. |
| [30] | M. Mastrogiacomo, S. Scaglione, R. Martinetti, et al., Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics, Biomaterials 27 (2006) 3230-3237. |
| [31] | Y. Arima, H. Iwata, Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers, Biomaterials 28 (2007) 3074-3082. |
| [32] | Y. Tamada, Y. Ikada, Effect of preadsorbed proteins on cell adhesion to polymer surfaces, J. Colloid Interface Sci. 155 (1993) 334-339. |
| [33] | L.J. Gibson, M.F. Ashby, The mechanics of three-dimensional cellular materials, Proc. Roy. Soc. A: Math. Phys. 383 (1982) 43-59. |
| [34] | K.G. Nair, A. Dufresne, Crab shell chitin whisker reinforced natural rubber nanocomposites. 2. Mechanical behavior, Biomacromolecules 4 (2003) 666-674. |




