Polymer/inorganic hybrid materials have received increasing interest in recent years for their favorable combination of the properties of both the inorganic particles and the polymer [1, 2]. The surface modification of solid particles is a kind of method to prepare polymer/inorganic hybrid materials. There are two ways to attach polymers chains to the surface of inorganic particles [3, 4]: ‘‘grafting to’’ and ‘‘grafting from’’ techniques. In the ‘‘grafting to’’ method,the end-functional polymer chains react with the surface groups on the substrate. The disadvantage of this method is the reactivity and surface graft densities of the polymer chains are lower. In the ‘‘grafting from’’ approach,a variety of polymers can easily graft from the surface of the inorganic particles with a higher graft density.
Some authors have proposed a combination of the previously described methods with surface-initiated living radical polymerization (SI-LRP) to synthesize well-defined polymer brushes on inorganic material surfaces [5, 6]. Two of the most well-established living radical polymerization techniques are atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer (RAFT). RAFT has been proven to be an extremely versatile controlled free radical polymerization technique,applicable to a wide variety of monomers,affording polymers with predetermined molecular weights and narrow polydispersity. For example,Chen and coworkers [7] demonstrated a novel,efficient postmodification route for preparation of smart hybrid SiO2 nanoparticles with a stimuli-responsive nanoshell based on RAFT and click chemistry. They also observed that the grafting density of polymer chains can be easily adjusted by changing the reaction time. Zhao et al. [8] reported on the use of alkoxysilane functionalized RAFT agents for surface modification of graphene oxide (GO),and homopolymers and diblock copolymers grafted GOs were obtained by a tandem approach comprising simultaneous hydroxyl-alkoxysilane coupling reaction and RAFT process. We [9] also applied this technique in a previous work to prepare a novel rare earth optical-functional hybrid material based on reversible addition-fragmentation chain transfer (RAFT) polymerization. After immobilization of the trithiocarbonate on hollow sphere (HS) surface,poly(methyl methacrylate) (PMMA) and Tb complex were successfully grafted from the surface of hollow spheres. Fluorescence spectra confirmed that the HS-gPMMA-b-Tb hybrid materials exhibited strong fluorescence properties.
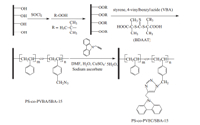
Due to the robust structures and well-ordered arrays of its uniform nanometer-sized channel pores,mesoporous silica SBA-15 has gathered considerable attention as a hard template for synthesis of nanoparticles,nanowires,and nanowire networks [10, 11, 12]. In this study,we wish to report the first successful use of surface initiated RAFT copolymerization combined with the ‘‘click’’ chemistry technique to synthesize controlled copolymers with fluorescence properties in mesoporous silica SBA-15 channel pores. The hydroxyl groups in mesoporous silica SBA-15 channel pores were converted into peroxide groups and were further used to mediate the RAFT copolymerization of styrene and 4-vinylbenzyl azide. Then,the copolymer with fluorescence properties in mesoporous silica SBA-15 channel pores was formedvia‘‘click’’ reaction. The reaction schemes are listed in Fig. 1.
|
Download:
|
| Fig. 1. Synthetic pathway for mesoporous silica SBA-15 supported copolymers with fluorescence properties via RAFT polymerization and ‘‘click’’ chemistry. | |
Materials and reagents: S,S'-Bis(α,α-dimethyl-α'' -acetic acid)-trithiocarbonate (BDAAT),mesoporous silica SBA-15,SBA-15 with peroxide groups,4-vinylbenzyl azide (VBA),andN-propargylcarbazole (PC) were synthesized according to the related literature procedures,respectively [13, 14, 15, 16, 17]. According to the detecting method of the literature [15],the content of peroxide groups in SBA-15 substrate is 0.9 mmol g-1. All other chemical reagents were of analytical grade and used without further purification.
Synthesis of polystyrene-co-poly(4-vinylbenzyl azide) copolymer in mesoporous silica SBA-15 (PS-co-PVBA/SBA-15): A mixture of peroxide-functionalized SBA-15 (0.3 g,0.027 mmol peroxide groups),styrene (3 mL,0.027 mol),4-vinylbenzyl azide (VBA) (0.3 mL,2 mmol),BDAAT (0.01 g,0.035 mmol),and cyclohexanone (3 mL) was added to a 50 mL dry flask equipped with a magnetic stirrer. The contents were purged with nitrogen for 10 min to eliminate the oxygen,and then the flask was placed into an oil bath and heated to 65°C for 48 h. After the reaction,the flask was cooled in an ice water bath and opened. The reaction mixture was diluted with THF and separated by centrifugation. The crude product was precipitated in a large excess of methanol and dried at 40°Cin vacuum,yielding a yellow powder quantitatively. The SBA-15 with grafted copolymers of styrene and 4-vinylbenzyl azide (PS-coPVBA/SBA-15) was separated from the mixture followed by Soxhlet’s extraction with THF. After completely removing the chemisorbed/ungrafted copolymers,the grafted material PS-coPVBA/SBA-15 (0.34 g) was dried at 40°C in vacuum. The grafted copolymer content in PS-co-PVBA/SBA-15 can be calculated from the weight difference of the resulting PS-co-PVBA/SBA-15 and the SBA-15 (0.3 g),that is 11.8%.
Preparation of copolymers with fluorescence properties in mesoporous silica SBA-15 (PS-co-PVBC/SBA-15) via ‘‘click’’ reaction: PS-co-PVBA/SBA-15 (0.10 g) and N-propargyl-carbazole (0.15 g,0.73 mmol) were dispersed in 3 mL of DMF. A solution of CuSO4·5H2O (5.7 mg,22.8mmol) in 0.2 mL of water was added to the mixture followed by addition of a freshly prepared solution of sodium ascorbate (9.0 mg,45.5mmol) in 0.2 mL of water. The mixture was heated in an oil bath at 40°C overnight under argon atmosphere. At the end of the reaction,the mixture was filtrated and washed with a large excess of water to remove all the Cu II trapped inside the polymer chains. To remove the excess Npropargyl-carbazole,the product was precipitated in excess of hexane. Copolymers grafted SBA-15 hybrid materials with light blue color were obtained by filtering and drying under vacuum at room temperature.
Characterization: The structure of the samples was characterized by Nicolet AVATAR 360 Fourier transform infrared spectroscopy (FT-IR) from 400 cm-1 to 4000 cm-1 by the KBr pellet method. Transmission electron microscopy (TEM) analysis was performed on a JEOL JEM-1400 transmission electron microscope. The thermal decomposition behavior of the materials was examined by means of thermogravimetry analysis (TGA) with a heating rate of 10°C min-1 in nitrogen atmosphere on a model STA 449C simultaneous DSC-TGA (Netzsch Instruments,Germany). The elemental analyses (EA) were performed on a GmbH VarioEL elemental analysis system. XRD patterns were recorded on a German Bruker’s D8 Advanced powder diffractometer using Cu Ka radiation. N2adsorption-desorption isotherms were undertaken at 77 K on a NOVA4000e Automated Surface Area Analyzer. The samples were out gassed at 393 K for 6 h under nitrogen before measurement. The BET specific area was evaluated with the BET method and determined in the partial pressure (P/P0) range 0.05- 0.35,and total pore volume was analyzed following BJH (Barrett- Joyner-Halenda) algorithm and determined from the amount of the nitrogen adsorbed at a relative pressure of 0.99. Fluorescence excitation and emission spectra were recorded on an Edinburgh Instruments FLS920 spectrofluorimeter from a 450 W stable xenon lamp. 3. Results and discussion 3.1. RAFT copolymerization of styrene and 4-vinylbenzyl azide inside
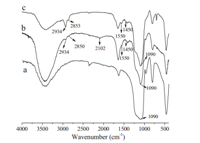
mesoporous silica SBA-15 The PS-co-PVBA/SBA-15 hybrid materials were prepared using RAFT polymerization. The samples were characterized by FT-IR, TEM,and TGA. Fig. 2 shows the infrared spectra of SBA-15,PS-coPVBA/SBA-15 and PS-co-PVBC/SBA-15. The strong absorption peak at 1090 cm-1 can be attributed to Si-O stretching vibration of SBA-15. As for Fig. 2b,the peaks at 3000-2800 cm-1 resulted from the C-H stretching vibrations and the peaks at 1560- 1450 cm-1 came from C55C stretching vibrations of the benzene ring,and the peak at 2102 cm-1 represented the azide groups of 4-vinylbenzyl azide. Moreover,the signal at 2102 cm-1 disappeared after the ‘‘click’’ reaction (Fig. 2c),indicating high efficiency of the ‘‘click’’ reaction between SBA-15-g-PS-co-PVBA andN-propargyl-carbazole.
|
Download:
|
| Fig. 2. FT-IR spectra of SBA-15 (a), PS-co-PVBA/SBA-15 (b) and PS-co-PVBPC/SBA-15(c). | |
Fig. 3 shows the typical TEM images of PS-co-PVBA/SBA-15 composites. As shown in Fig. 3,well-defined 2D-hexagonal symmetry is clearly seen,but the pore surfaces are rough,and some amorphous copolymers even plug the nanochannels,which is due to the copolymerization of styrene and 4-vinylbenzyl azide inside the channel pores of SBA-15.

|
Download:
|
| Fig. 3. TEM images of PS-co-PVBA/SBA-15 viewed along the pore axis (a) and perpendicular to the pore axis (b). | |
The content data of copolymers inside channel pores of SBA-15 was obtainedviaTGA. Fig. 4 displays the TGA curves of SBA-15 and PS-co-PVBA/SBA-15. The data show three weight loss zones associated with the evaporation of excess H2O molecules under 100°C (zone I),organic groups on SBA-15 (zone II),and the remaining inorganic salts (zone III). The organic content can be determined from the difference in weight loss between 100 and 500°C. The polymer content calculated for the hybrid material is relatively high (13.2 wt%) which agrees with the value calculated by weighing.

|
Download:
|
| Fig. 4. TGA curves of SBA-15 (a) and PS-co-PVBA/SBA-15 (b). | |
The nitrogen sorption isotherms and pore size distributions of SBA-15 and PS-co-PVBA/SBA-15 are shown in Fig. 5. The corresponding porous characteristics are listed in Table 1. The SBA-15 and the PS-co-PVBA/SBA-15 hybrid materials basically show IV-type curves with the classical hysteresis loop,which is typical of hexagonal cylindrical mesoporous materials. It shows that PS-co-PVBA/SBA-15 still maintained the cylindrical structure and demonstrates that the modifying of SBA-15 by the copolymer does not deteriorate the ordered pore structure. The surface area and pore volume decreased from 417 m2 g-1 and 0.949 cm3 g-1 for SBA-15 to 291 m2 g-1 and 0.680 cm3 g-1 for PS-co-PVBA/SBA-15, indicating that the SBA-15 has been modified by copolymer. Moreover,the BJH pore size also decreased from 21.2 nm to 17.5 nm. This result shows that PS-co-PVBA/SBA-15 has a significant effect on the surface area and pore volume.

|
Download:
|
| Fig. 5. N2 adsorption–desorption isotherms (a) and pore size distributions (b) of samples. | |
| Table 1 Textural properties of SBA-15 and PS-co-PVBA/SBA-15. |
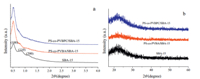
Fig. 6a shows small-angle XRD patterns of pure SBA-15,PS-coPVBA/SBA-15 and PS-co-PVBC/SBA-15. All samples show three well resolved peaks indexed to (1 0 0),(1 1 0) and (2 0 0) reflections associated with p6 mm hexagonal symmetry which belongs to a three-dimensional mesoporous cellular foam (MCF) structure [18]. The wide-angle XRD patterns of pure SBA-15,PS-coPVBA/SBA-15 and PS-co-PVBC/SBA-15 are shown in Fig. 6b. The only one broad band between 2θ=20° and 30° is the characteristic band of amorphous silica walls of the pristine material,evidencing that the encapsulated copolymer in the channels is amorphous [19].
|
Download:
|
| Fig. 6. XRD (a) Low- and (b) wide-angle X-ray diffraction patterns of SBA-15, PS-co-PVBA/SBA-15 and PS-co-PVBPC/SBA-15. | |
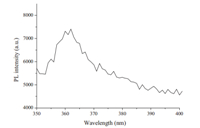
As mentioned above,the copolymer preparedviathe RAFTmediated process has azide groups. Thus,the PS-co-PVBA/SBA-15 hybrid materials can couple withN-propargyl-carbazole (PC)via ‘‘click’’ reaction for further functionalization. After ‘‘click’’ reaction for 24 h on PS-co-PVBA/SBA-15 substrate in DMF medium,the copolymer brushes with fluorescence properties in SBA-15 (PS-coPVBC/SBA-15) come into being. The fluorescence properties of the sample were determined by spectrofluorimeter. Fig. 7 shows the fluorescence spectra of PS-co-PVBC/SBA-15 hybrid materials. The excitation spectrum is dominated by a dominant broad band from250 to 330 nm in a narrow region with the maximum peak at 296 nm. These excitation spectra bands are the effective absorption for the luminescence of carbazole. As a result,the strong blue luminescence is observed,and the emission peak at 362 nm is assigned to the partially overlapped excimer of the carbazole moieties. The fluorescence properties analysis results show that theN-propargyl-carbazole has reacted with azide groups on PS-coPVBA/SBA-15 substratevia‘‘click’’ chemistry.
|
Download:
|
| Fig. 7. The emission spectra of PS-co-PVBPC/SBA-15 hybrid material. Excitation wavelength: 296 nm. | |
The controlled copolymerization in mesoporous silica SBA-15 was investigated in this work. After immobilization of the peroxide groups on SBA-15,PS-co-PVBA copolymer chains were successfully copolymerized inside mesoporous silica SBA-15. Moreover,the azide groups of PS-co-PVBA in SBA-15 reacted withN-propargylcarbazole,yielding PS-co-PVBC/SBA-15 hybrid material. Fluorescence spectra confirmed that the PS-co-PVBC/SBA-15 hybrid materials exhibited strong fluorescence properties. This work presented a new method to synthesize copolymers with fluorescence properties in mesoporous silica SBA-15.
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China (No. 21203085) and Promotive Research Fund for Young and Middle-aged Scientists of Shandong Province in China (doctor fund,Nos. BS2011CL011,BS2011CL012 and BS2012CL009).
| [1] | O. Kir, W.H. Binder, Living anionic surface initiated polymerization (LASIP) of isoprene from silica nano-and glass particles, Eur. Polym. J. 49 (2013) 3078-3088. |
| [2] | L.P. Wang, Y.P. Wang, K. Yuan, Z.Q. Lei, Synthesis and characterization of surfaceinitiated polymer brushes on silicon substrates by reversible addition fragmentation chain transfer polymerization, Polym. Adv. Technol. 19 (2008) 285-290. |
| [3] | S. Berger, A. Synytska, L. Ionov, K.J. Eichhorn, M. Stamm, Stimuli-responsive bicomponent polymer janus particles by “grafting from”/“grafting to” approaches, Macromolecules 41 (2008) 9669-9676. |
| [4] | Q.S. Song, Y. Yang, K. Gao, H.H. Ma, Study on the novel rare-earth nanocrystals/PNIPAM complex hydrogels prepared by surface-initiated living radical polymerization, J. Lumin. 136 (2013) 437-443. |
| [5] | X.H. Lv, L.P. Wang, G. Li, et al., Preparation and characterization of optically functional hollow sphere hybrid materials by surface-initiated RATRP and “click” chemistry, Chin. Chem. Lett. 24 (2013) 335-337. |
| [6] | L.P. Wang, L.H. Dong, J.C. Hao, et al., Fabrication of block copolymer brushes on hollow sphere surface via reverse iodine transfer polymerization, J. Colloid Interface Sci. 361 (2011) 400-406. |
| [7] | J. Chen, M. Liu, C. Chen, H. Gong, C. Gao, Synthesis and characterization of silica nanoparticles with well-defined thermoresponsive PNIPAM via a combination of RAFT and click chemistry, ACS Appl. Mater. Interface 3 (2011) 3215-3223. |
| [8] | K. Jiang, C.N. Ye, P.P. Zhang, X.S. Wang, Y.L. Zhao, One-pot controlled synthesis of homopolymers and diblock copolymers grafted graphene oxide using couplable RAFT agents, Macromolecules 45 (2012) 1346-1355. |
| [9] | L.P. Wang, L.M. Zhao, W.Z. Li, Fabrication of polymer-hollow sphere opticalfunctional hybrid material via RAFT polymerization, React. Funct. Polym. 70 (2010) 35-40. |
| [10] | V. Subbaramaiah, V.C. Srivastava, I.D. Mall, Optimization of reaction parameters and kinetic modeling of catalytic wet peroxidation of picoline by Cu/SBA-15, Ind. Eng. Chem. Res. 52 (2013) 9021-9029. |
| [11] | K. Mantri, P.R. Selvakannan, J. Tardio, S.K. Bhargava, Synthesis of very high surface area Au-SBA-15 materials by confinement of gold nanoparticles formation within silica pore walls, Colloid Surf. A 429 (2013) 149-158. |
| [12] | P. Zarabadi-Poor, A. Badiei, A.A. Yousefi, J. Barroso-Flores, Selective optical sensing of Hg(Ⅱ) in aqueous media by H-acid/SBA-15: a combined experimental and theoretical study, J. Phys. Chem. C 117 (2013) 9281-9289. |
| [13] | J. Zhou, L. Wang, Q. Yang, et al., Novel thermoresponsive and pH-responsive aggregates from self-assembly of triblock copolymer PSMA-b-PNIPAAm-b-PSMA, J. Phys. Chem. B 111 (2007) 5573-5580. |
| [14] | J. Han, P. Fang, W. Jiang, L. Li, R. Guo, Ag-nanoparticle-loaded mesoporous silica: spontaneous formation of ag nanoparticles and mesoporous silica SBA-15 by a one-pot strategy and their catalytic applications, Langmuir 28 (2012) 4768-4775. |
| [15] | Y.P. Wang, X.W. Pei, X.Y. He, K. Yuan, Synthesis of well-defined, polymer-grafted silica nanoparticles via reverse ATRP, Eur. Polym. J. 41 (2005) 1326-1332. |
| [16] | K.Malagu, P.Gué rin, J.C.Guillemin, Preparationof solublepolymericsupportswith a functional group for liquid-phase organic synthesis, Synlett 2 (2002) 316-318. |
| [17] | C.H. Li, X.F. Liu,M.J. Yuan, et al., Unusual fluorescence enhancement of a novel carbazolyldiacetylene bound to gold nanoparticles, Langmuir 23 (2007) 6754-6760. |
| [18] | H. Zhao, W. Li, M. Du, X. Pu, X. Shao, A facile strategy to synthesize spherical SBA-15 silicas by the addition of poly(vinyl alcohol), Mater. Lett. 92 (2013) 33-35. |
| [19] | B.S. Tian, C. Yang, Temperature-responsive nanocomposites based on mesoporous SBA-15 silica and PNIPAAm: synthesis and characterization, J. Phys. Chem. C 113 (2009) 4925-4931. |





