bInstitute of Bioresources and Sustainable Development, Department of Biotechnology, Imphal 795001, India;
cDepartment of Life Sciences, Manipur University, Imphal 795003, India;
dChemical Engineering and Process Development, National Chemical Laboratory, Pune 411008, India
Nanotechnology has attracted global attention because materials at nano-scale have properties unique from their bulk equivalents. As the size of materials is reduced to the nanometer regime the resulting properties change noticeably. Considerable efforts have been made to characterize and describe the physical and chemical properties of metal oxide nanomaterials because of their significant applications in numerous technological fields [1, 2]. The oxides of transition metals are an important class of semiconductors that have wider applications in magnetic storage media,solar energy transformation,electronics,catalysis,etc.[3, 4, 5, 6]. Among various transition metal oxides,copper oxide (CuO) has attracted greater attention due to its fascinating properties such as the basis of high critical temperature (Tc) superconductors. CuO is a semiconducting compound with a narrow band gap and is used for photoconductive and photothermal applications [7]. An improved understanding of nanoparticles and biological cell interactions can lead to the development of new sensing,diagnostic,and treatment capabilities,such as improved targeted drug delivery,gene therapy,magnetic resonance imaging (MRI) contrast agents,and biological warfare agent detection [8, 9, 10]. Nano-sized metallic copper and its oxides possess good potential for photo-catalytic [11],sensing applications [12] and their usage in bio-related fields including fouling control and nano-toxicology [13] is also being explored.
Nanotoxicology was proposed as a new branch of toxicology to address the gaps in knowledge and to specifically address the adverse health effects likely to be caused by nanomaterials [14]. Nanotoxicology is involved in proposing reliable,robust,and dataassured test protocols for nanomaterials in human and environmental risk assessment [15]. With the increasing demand of nanomaterials in the field of biological applications,cytotoxicity of the nanomaterials becomes a major concern. Copper oxide nanoparticles appeared to have greater cytotoxicity compared to their bulk counterpart and to other metal oxides nanoparticles [16].
Recently,our research group has reported the preparation of nanomaterials using polyethylene glycol (PEG) along with glycerol as the capping agent cum medium [17, 18]. The importance of using such long chain hydrocarbon in the synthesis of nanomaterials is the formation of monodispersed nanomaterials. Apart from that, PEG is also considered as one of the best non-ionic polymer used in biomedical science especially in the delivery of anticancer drugs and other bioactives clinically. It is considered as a versatile candidate for the prodrug conjugation due to its high solubility in the aqueous medium. The covalent attachment of PEG (PEGylation) of peptides proteins,drugs,and bioactives is known to enhance the aqueous solubility of hydrophobic drugs,prolong circulation time,minimize nonspecific uptake,and achieve specific tumor targetability through the enhanced permeability and retention effect. Numerous PEG-based therapeutics have been developed,and several have received market approval. A vast amount of clinical experience has been gained which has helped to design PEG prodrug conjugates with improved therapeutic efficacy and reduced systemic toxicity [19].
In the present study,CuO nanomaterials were prepared by a novel synthesis technique using PEG-Glycerol as the capping agent cum medium and their applicability in the field of biological science have been studied. Detail characterization and the effect of annealing on the properties of CuO nanomaterials have also been investigated. The work further investigate how CuO nanoparticles interact with human cervical cancer cell (HeLa) and three human urolithiasis inducing bacteria such asEscherichia coli(MTCC 729), Proteus mirabilis(MTCC 425) and Klebsiella pneumoniaesub sp. pneumoniae(MTCC 432). 2. Experimental
CuO nanomaterials were prepared by a relatively novel synthesis route where PEG is used as a capping agent and EG as the solution medium. The prepared nanomaterials were annealed at higher temperature to analyze the effect of annealing temperature on the properties of the synthesized nanomaterials. Detail experimental procedure and analysis about the phase formation of the CuO nanomaterials along with IR studies (Fig. 1) are shown in Supporting information.

|
Download:
|
| Fig. 1. (a) XRD spectra (along with PCPDF Card No. 80-0656 for Cu(OH)2 and 80-1917 for CuO) and (b) IR spectra of bulk and CuO nanoparticles (as-prepared and 700°C annealed samples). | |
The thermal properties of as-prepared Cu(OH)2nanoparticles were investigated by thermogravimetric analysis (TGA) and differential thermal analysis (DTA),as shown in Fig. 2a. The initial weight loss of~45% in the TGA curve up to 220°C,accompanied by an endothermic peak at 170°C,is assigned to the loss of free and coordinated water molecules present in the sample [17, 18]. The further changes in weight (~15%) in the temperature range of 220- 570°C in the TGA curve is due to the loss of capping agent (PEG) and glycerol. It should be noted that the apparent increase in weight (TGA curve) above 580°C is due to an artifact of the instrument. No change in the TGA curve is observed above 650°C, which indicates the formation of stable CuO nanoparticles. 3.2. Absorption characterization
The electronic spectra measurement of freshly prepared aqueous solutions of as-prepared,Cu(OH)2 and 700°C annealed samples of CuO were carried out in the region 200-900 nm (Fig. 2b). The spectra of as prepared CuO nanoparticles show the charge transfer band in the UV region at 247 nm while that of the annealed sample does not show any band within these ranges. Similar observation has also been reported in other semiconductor compounds where there is a variation in the band edge absorption on annealing [20, 21]. Hong et al.have reported that there is a change of the optical band gap of ZnO thin films on annealing which is possibly due to the increase in the grain size [20]. Bao et al. has also further clarified about the change in the band gap on annealing at higher temperatures for SrTiO3 thin films and suggested that a shift of the energy gap was due to both the quantum-size effect and the existence of the amorphous phase in the thin films [21]. In our work,the loss of the band edge absorption on annealing may be attributed to the loss of the water molecules as well as the increase in the particle grain size thereby leading to the increase crystallinity of the sample. Earlier diffraction studies also reveal the same observation,where the amorphous nature of the as-prepared sample loses on annealing giving a more crystalline nanoparticle.

|
Download:
|
| Fig. 2. (a) Thermal analysis (TGA-DTA) along with (b) UV–vis spectra of CuO nanoparticles. | |
Fig. 3 shows the TEM images of as-prepared Cu(OH)2 and annealed samples of CuO nanoparticles at different magnifications. The particle sizes of the as-prepared nanomaterials were found to be spherical in shape and are in the range of 50-80 nm. The individual particles are well separated and clear boundaries of the nanomaterials were observed. The particles are found to be agglomerated on annealing thereby slightly increasing the size of the CuO nanomaterials. The particle sizes calculated from the XRD data and TEM images are found to be in good agreement. The evaporation of the capping agent,PEG and glycerol on annealing lead to the agglomeration of the nanomaterials thereby enhanced the crystallinity of the sample. The SAED patterns further indicates the amorphous and crystalline nature of as-prepared,hydrated Cu(OH)2 nanomaterials and annealed CuO nanomaterials,respectively (Fig. S1 in Supporting information)

|
Download:
|
| Fig. 3. TEM images of (a, b) as-prepared Cu(OH)2 and (c, d) 700°C annealed CuO nanoparticles. | |
Fig.S2 in Supporting information shows the excitation and emission spectra of as prepared (Cu(OH)2) and annealed sample of bulk CuO. The excitation spectra of the bulk sample show a broad absorption peak at 320-330 nm. On excitation at 320 nm,it gives a broad peak in the range of 390-430 (centered on ~395 nm) and a small hump centered at~487 nm. The emission centered around ~398 nm may be due to the phonons engendered by the trapped charge carriers generated by crystal lattice defects induced by strain or dangling bonds at the surface induced crystal lattice defects [22]. The emission for the bulk sample is mainly due to the band edge emission. On heating,the intensity of the peaks was drastically reduced.
Similar reduction in the luminescence intensity was also observed for the CuO nanoparticles (Fig. S3 in Supporting information). Here,emission at 487 nm was observed with a sharp peak along with an absorption peak at 360 nm for the asprepared samples,Cu(OH)2nanomaterials. The 360 nm absorption can be assigned as the band edge absorption of Cu(OH)2 nanoparticles. A small hump centered on 530 nm was also observed. This small hump arises from the singly ionized oxygen vacancy resulting in green emission of Cu(OH)2materials because of recombination of a photo generated hole with a singly ionized electron in valence band [23]. With annealing,intensity is reduced but emission peaks can still be observed. The band edge emission is observed in the range of 420 nm for the annealed samples. The photoluminescence properties of nanomaterials can be utilized in the field of biologyviz.Bioimaging [24]. 3.5. Biological applications 3.5.1. Cytotoxicity test: effects of test samples on the proliferation of HeLa cells
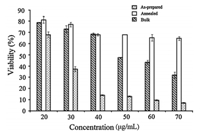
Fig. 4 shows the percentage of viable HeLa cells after treatment with the samples. The relative number of surviving cells decreased in a dose-dependent manner. The highest antiproliferative activity was found in the as-prepared nanoparticles (P<0.01) followed by bulk and annealed samples,although there was no significant difference observed between the three lowest concentrations in bulk and annealed samples. For instance,as-prepared nanoparticles at 20-70mg/mL decreased the proliferation of HeLa cells by 68%-7%. On the other hand,20-70mg/mL of the bulk and annealed sample,each decrease the viable cells by 78%-32% and 81%-64%, respectively. Correspondingly,the IC50 value of the as-prepared Cu(OH)2 sample (22.4±0.7mg/mL) was found to be the lowest compared with the bulk (52.7±2.7mg/mL) and annealed sample (113.8±6.2mg/mL) (P<0.05).
|
Download:
|
| Fig. 4. Cytotoxicity effect of the as-prepared, annealed nanomaterials and bulk samples against HeLa cells. Cell survival was determined as the percentage of the control from three independent experiments. Each bar represents the mean±SD (n = 3). Bars marked without a common letter within each concentration are significantly different (P<0.01). | |
The possible reason for the inhibitory effect on the proliferation of cells is not understood completely but very recently,Sun et al.[25] have reported the cytotoxicity effect of a series on metal oxide nanomaterials like CuO,SiO2,Fe2O3,Fe3O4 and TiO2 on human adenocarcinoma A549 cells in which they suggested that autophagy may play a crucial role in metal oxide nanomaterials induced cell death. Autophagy or cellular self-congestion is the process in which cell degrade protein and organelles by engulfing them in double membrane vacuoles known as autophagosomes [26]. In their work,they have mentioned that CuO have the highest cytotoxicity as compared with the other metal oxide nanomaterials under study.
The bulk and as-prepared nanomaterials are in hydrated form (Cu-OH),while that of the annealed sample is in the pure form of CuO (as already explained in the XRD analysis above). The high cell death in case of the as-prepared sample correlates with that of the reported work which indicates that CuO have high cytotoxicity. In case of the CuOH (as-prepared and bulk samples),the possibility of releasing of Cu2+ ions is high as compared to the annealed CuO samples as the Cu-OH bond is easily ionizable w.r.t. the Cu==O bond. The presence of Cu2+ ions in the cell may lead to protein denaturation,disorganize helical structure and damage important biological processes which ultimately lead to cell death. On the other hand,the presence of capping agent PEG further enhances the cell death [27]. Corpet et al.have indicated that polyethylene glycol (PEG) has remarkable efficacy as a chemopreventive agent [28]. It has been further reported that PEG has the potential to suppress adenomas in humans [29],colonic epithelial proliferation [30]. Besides the long hydrocarbon chain length acts as the perfect carrier of the nanomaterials. Researcher has also report the possibility of the generation of toxic reactive oxygen species (ROS) from the defect in the lattices of metal oxides in aqueous suspension,which lead to the cell death [31, 32]. The decrease in the cytotoxicity capabilities of the annealed sample may be due to the reorganization of the crystal lattice and the loss of defects which in turn reduces the generation of the ROS. Collectively, all these effects lead to the high cytotoxicity capabilities of the as-prepared samples as compared with that of the bulk and annealed samples. 3.5.2. Antibacterial activity
Fig. 5 shows the results of the antibacterial activity of the bulk CuO,as-prepared and annealed CuO nanoparticles along with positive control antibiotic gentamycin against the three selected bacteriaE. coli,P. mirabilisandK. pneumoniaesub sp.pneumoniae.It has been observed from the data that,as compare to positive control antibiotic gentamycin,all the tested samples shows fair antibacterial activity against E. coli and P. mirabilis but less antibacterial activity againstK. pneumoniaesub sp.pneumoniae. The variation in the sensitivity or resistance could be due to the differences in the cell structure,physiology,metabolism,or degree of contact of organisms with nanoparticles. It has been reported that K. aerogenes strains are resistant to a wider range of antibiotics thanE. colistrains [33]. It is also reported thatKlebsiellacells have a thick coat of slime or extracellular polysaccharide [34]. The decrease in the antimicrobial activity of the tested samples against K. pneumoniaesub sp.pneumoniae might be due to the presence of such polysaccharide coat. Differential nanoparticles diffusion rates may also occur in these three bacterial cells.

|
Download:
|
| Fig. 5. Bar graph showing the antibacterial activity of as-prepared, annealed CuO nanoparticle and bulk samples along positive control antibiotic gentamycin against the human pathogenic bacteria (A)Escherichia coli, (B) Proteus mirabilisand (C)Klebsiella pneumoniaesub sp.pneumoniae, recorded at the end of 24 h. | |
The inhibition of the bacterial growth was also found to be increased with increasing sample concentrations although there was no significance difference observed between the concentrations (7.50 and 3.75 mg/mL) for the bulk sample against theE. coli bacteria. The data further show the enhancement in activity of the as-prepared CuO nanoparticles as compared to the activity of the bulk and annealed samples. The minimum inhibition concentrations (MIC) of as-prepared Cu(OH)2 nanomaterials,bulk and annealed samples of CuO samples forE. coliandP. mirabiliswere found to be 1.0,3.75,and 7.5 mg/mL respectively. Fig. S4 in Supporting information shows the photographs of diameter of zone of inhibition of the as-prepared,annealed CuO nanoparticles and bulk samples for the concentrations (15.0,7.50 and 3.75) mg/mL along with gentamycin antibiotic against the tested bacteria. The trend of antibacterial activity of the samples is also in close agreement with the result found in the above cytotoxicity analysis against the HeLa cell. The possible reason for higher bacterial growth inhibitory effect of as-prepared Cu(OH)2nanoparticles and bulk than that of annealed sample is already explained in the cytotoxicity analysis. Further enhancement of the inhibitory effect of the as-prepared Cu(OH)2nanoparticles on the growth of the bacteria is due to the large surface area of the Cu(OH)2 nanomaterials as compared with that of the bulk samples. The larger surface area of the nanomaterials allows the maximum interaction of the nanomaterials with the bacteria thereby restricting their growth rates. Sometimes even giving a long lasting antibacterial activity as recently reported for TiO2 nanotubes [35].
A few studies have been performed to elucidate the mechanism of the effect of nanoparticles on bacterial cells. It has been reported that copper ions released by the nanoparticles may attach to the negatively charged bacterial cell wall and rupture it,thereby leading to protein denaturation and cell death [36]. Copper ions inside the bacterial cells may bind to deoxyribonucleic acid molecules and become involved in cross-linking within and between the nucleic acid strands,resulting in the disorganized helical structure. In addition,copper ion uptake by the bacterial cells has also been found to damage important biochemical processes,there by leading to an overall diminishing in their growth rate [37, 38]. Nevertheless,further studies are required to confirm this and it is beyond the scope of this manuscript. 4. Conclusion
CuO nanoparticles were synthesized by PEG-Glycerol route and the effect of annealing on the properties of CuO nanoparticles with that of the bulk counterpart were investigated. The as-prepared samples were found to be Cu(OH)2which on annealing gives CuO nanoparticles. The morphology and the photoluminescence properties of the CuO nanoparticles were thoroughly studied. Preliminary studies on the biological systems were performed to determine if nanoparticles affect the growth of microbial cells in the presence of inorganic nanoparticles. The cytotoxicity test on the representative test samples indicate that the as-prepared Cu(OH)2 nanoparticles capped with PEG show the highest cytotoxicity compared to their bulk and annealed samples. The antibacterial activity further indicates the enhancement of antimicrobial effect of the as-prepared nanoparticles than that of their bulk and annealed samples against the E. coliand P. mirabilis.
AcknowledgmentsWe thank the University Grants Commission (UGC),New Delhi, India,for the award of Fellowship in Science for Meritorious Students (RFSMS) to one of the authors (Ayekpam Bimolini Devi) under the UGC-SAP-DRS programme.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.07.014.
| [1] | R.S. Devan, R.A. Patil, J.H. Lin, Y.R. Ma, One dimensional metal oxide nanostructures: recent developments in synthesis, characterization and applications, Adv. Funct. Mater. 22 (2012) 3326-3370. |
| [2] | H. Zhu, F. Zhao, L.Q. Pan, et al., Structural and magnetic properties of Mn-doped CuO thin films, J. Appl. Phys. 101 (2007) 09H111. |
| [3] | B.O. Regan, M. Gratzel, A low-cost, high-efficiency solar cell based on dyesensitized colloidal TiO2 films, Nature 353 (1991) 737-740. |
| [4] | M.N. Luwang, Microemulsion mediated synthesis of triangular SnO2 nanoparticles: luminescence application, Appl. Surf. Sci. 290 (2014) 332-339. |
| [5] | P.L. Singh, M.N. Luwang, S.K. Srivastava, Luminescence and photocatalytic studies of Sm3+ ion doped SnO2 nanoparticles, New J. Chem. 38 (2014) 115-121. |
| [6] | R.T. Stuart, P. Pattanasattayavong, T.D. Anthopoulos, Solution processable metal oxide semiconductors for thin film transistor applications, Chem. Soc. Rev. 42 (2013) 6910-6923. |
| [7] | V. Javier, Molecular chemistry to the fore: new insights into the fascinating world of photoactive colloidal semiconductor nanocrystals, J. Phys. Chem. Lett. 4 (2013) 653-668. |
| [8] | W.C.W. Chan, S. Nie, Quantum dot bioconjugates for ultrasensitive nonisotopic detection, Science 281 (1998) 2016-2018. |
| [9] | C. Chouly, D. Pouliquen, I. Lucet, J.J.L. Jeune, P. Jallet, Development of superparamagnetic nanoparticles for MRI: effect of particle size, charge and surface nature on biodistribution, J. Microencapsulation 13 (1996) 245-255. |
| [10] | P. Couvreur, C. Dubernet, F. Puisieux, Controlled drug delivery with nanoparticles: current possibilities and future trends, Eur. J. Pharm. Biopharm. 41 (1994) 2-13. |
| [11] | Z.K. Zheng, B.B. Huang, Z.Y. Wang, et al., Crystal faces of Cu2O and their stabilities in photocatalytic reactions, J. Phys. Chem. C 113 (2009) 14448-14453. |
| [12] | J.T. Zhang, J.F. Liu, Q. Peng, X. Wang, Y.D. Li, Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors, Chem. Mater. 18 (2006) 867-871. |
| [13] | G. Ren, D. Hu, E.W.C. Cheng, et al., Characterisation of copper oxide nanoparticles for antimicrobial applications, Int. J. Antimicrob. Agents 33 (2009) 587-590. |
| [14] | K. Donaldson, V. Stone, C.L. Tran, W. Kreyling, P.J.A. Borm, Nanotoxicology, Occup. Environ. Med. 61 (2004) 727-728. |
| [15] | N. Lewinski, V. Colvin, R. Drezek, Cytotoxicity of nanoparticles, Small 4 (2008) 26-49. |
| [16] | G. Oberdö rster, E. Oberdö rster, J. Oberdö rster, Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles, Environ. Health Perspect. 113 (2005) 823-839. |
| [17] | M.N. Luwang, R.S. Ningthoujam, Jagannath, S.K. Srivastava, R.K. Vatsa, Effects of Ce3+ co-doping and annealing on phase transformation and luminescence of Eu3+ doped YPO4 nanorods: D2O solvent effect, J. Am. Chem. Soc. 132 (2010) 2759-2768. |
| [18] | M.N. Luwang, R.S. Ningthoujam, S.K. Srivastava, R.K. Vatsa, Disappearance and recovery of luminescence in Bi3+, Eu3+ co-doped YPO4 nanoparticles due to presence of water molecules up to 800 ℃, J. Am. Chem. Soc. 133 (2011) 2998-3004. |
| [19] | S.S. Banerjee, N. Aher, R. Patil, J. Khandare, Poly(ethylene glycol)-prodrug conjugates: concept, design, and applications, J. Drug Deliv. 2012 (2012) 103973. |
| [20] | R.J. Hong, J.B. Huang, H.B. He, Z.X. Fan, J.D. Shao, Influence of different posttreatments on the structure and optical properties of zinc oxide thin films, Appl. Surf. Sci. 242 (2005) 346-352. |
| [21] | D.H. Bao, X. Yao, N. Wakiya, K. Shinozaki, N. Mizutani, Band-gap energies of sol-gel-derived SrTiO3 thin films, Appl. Phys. Lett. 79 (2001) 3767-3772. |
| [22] | K.K. Dey, A. Kumar, R. Shanker, et al., Growth morphologies, phase formation, optical & biological responses of nanostructures of CuO and their application as cooling fluid in high energy density devices, RSC Adv. 2 (2012) 1387-1403. |
| [23] | P.H. Huh, J.Y. Yang, S.C. Kim, Facile formation of nanostructured 1D and 2D arrays of CuO islands, RSC Adv. 2 (2012) 5491-5494. |
| [24] | M.N. Luwang, S. Chandra, D. Bahadur, S.K. Srivastava, Dendrimer facilitated synthesis of multifunctional lanthanide based hybrid nanomaterials for biological applications, J. Mater. Chem. 22 (2012) 3395-3403. |
| [25] | T. Sun, Y. Yan, Y. Zhao, F. Guo, G. Jiang, Copper oxide nanoparticles induce autophagic cell death in A549 cells, PLoS ONE 7 (2012) e43442. |
| [26] | A. Bergmann, Autophagy and cell death: no longer at odds, Cell 131 (2007) 1032-1034. |
| [27] | R.Q. Hang, A. Gao, X.B. Huang, et al., Antibacterial activity and cytocompatibility of Cu-Ti-O nanotubes, J. Biomed. Mater. Res. Part A 102A (2014) 1850-1858. |
| [28] | D.E. Corpet, G. Parnaud, M. Delverdier, G. Peiffer, S. Tache, Consistent and fast inhibition of colon carcinogenesis by polyethylene glycol in mice and rats given various carcinogens, Cancer Res. 60 (2000) 3160-3164. |
| [29] | E. Dorval, J.M. Jankowski, J.P. Barbieux, et al., Polyethylene glycol and prevalence of colorectal adenomas, Gastroenterol. Clin. Biol. 30 (2006) 1196-1199. |
| [30] | H.K. Roy, D.P. Kunte, J.L. Koetsier, et al., Chemoprevention of colon carcinogenesis by polyethylene glycol: suppression of epithelial proliferation via modulation of snail/β-catenin signaling, Mol. Cancer Ther. 5 (2006) 2060-2069. |
| [31] | S. Jun, K. Emiko, K. Fumio, et al., Detection of active oxygen generated from ceramic powders having antibacterial activity, J. Chem. Eng. Jpn. 29 (1996) 627-633. |
| [32] | A. Guy, L. Anat, D. Rachel, et al., Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury, Adv. Funct. Mater. 19 (2009) 842-852. |
| [33] | Topley and Wilson's Principles of Bacteriology, Virology and Immunity, Williams and Wilkins, Baltimore, 1975, pp. 859-900. |
| [34] | E. Miftode, O. Dorneanu, D. Leca, et al., Antimicrobial resistance profile of E. coli and Klebsiella spp. from urine in the Infectious Diseases Hospital Iasi, Rev. Med. Chir. Soc. Med. Nat. Iasi 113 (2008) 478-482. |
| [35] | G. Ang, R. Hang, X. Huang, et al., The effect of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts, Biomaterials 35 (2014) 4223-4235. |
| [36] | Y.E. Lin, R.D. Vidic, J.E. Stout, C.A. McCartney, V.L. Yu, Inactivation of Mycobacterium avium by copper and silver ions, Water Res. 32 (1998) 1997-2000. |
| [37] | J.H. Kim, H. Cho, S.E. Ryu, M.U. Choi, Effects of metal ions on the activity of protein tyrosine phosphatase VHR: highly potent and reversible oxidative inactivation by Cu2+ ion, Arch. Biochem. Biophys. 382 (2000) 72-80. |
| [38] | S.J. Stohs, D. Bagchi, Oxidative mechanisms in the toxicity of metal ions, Free Radic. Biol. Med. 18 (1995) 321-336. |




