Catalyst-free chemical processes,which can eliminate the tedious preparation/recovery of catalyst and directly yield catalyst-free final products,have attracted growing interest as a green approach [1, 2]. Within the past decades,the investigation of green and sustainable chemistry has led to the development of cleaner and more environment-benign chemical process [3, 4]. And tens of thousands of articles have been published on the beneficial effect of microwave irradiation [5, 6, 7, 8]. In our previous work,ring opening insertion polymerization (ROIP) of e-caprolactone byH-phosphonate initiator was carried out under microwave irradiation [9]. The result inspired us to develop a polymerization reaction without catalyst by taking the advantages of microwave chemistry. Nowadays,enzymatic polymerization is great of interest as a new synthetic method for polymer synthesis,which has provided a convenient synthetic approach comparing with conventional methods [10, 11]. As a powerful enzyme catalyst,Novozyme-435 (N-435),lipase B from Candida antarctica immobilized on macroporous acrylic resin,has shown many successful examples in polymer synthesis [12, 13, 14].
Since the first successful isolation of a stable singlet carbene by Arduengoet al. [15],carbene chemistry has attracted considerable attention in polymer synthesis and has proved to be an attractive method to construct polymers with unique architectures that are difficult to synthesize by traditional approaches [16, 17, 18, 19]. The first example of carbene polymerization using allyl diazoacetate as the precursor and copper as the catalyst was reported in 2002,and after that,polymerizations of various diazo compounds with the assistance of multiple catalysts have been published [20, 21, 22]. The polymerizations of diazoketone and diazocarbonyl derivatives by palladium [23],organoaluminum [24],rhodium [22, 25],andNheterocyclic carbene (NHC) have been studied in detail [26]. Additionally,Lewis acids and transition metallic complexes have also been utilized as the catalytic species [27, 28, 29, 30, 31, 32]. It has been reported that poly(carbethoxycarbene) (PCEC) was obtained from ethyl diazoacetate (EDA,N2CHCO2CH2CH3) by two steps: the nitrogenelimination of EDA to generate carbethoxycarbene (CEC) first and then the polymerization of CEC,which were catalyzed by Lewis acidic mediators (borane compounds and functionalized sulfurylides) and transition metal complexes (Cu,Pd and Rh) [28, 30]. However,without exception,the formation and polymerization of the carbenes from diazo compounds proceeded in the presence of catalyst in the published works.
Although many examples of the azo group’s (-N==N-) incorporation into polymerization have been reported,the structures of the polymerization products and the reaction mechanism are unidentified [26, 33]. It should be noted that the azo-incorporated oligomers,as well as those obtained by many catalysts other than rhodium,are generally atactic,with low number-average molecular weight (Mn). However,many of them have an ill-defined structure [23, 24, 28]. Moreover,the way in which the azo group is incorporated into the polymer main chain and the accurate repeating units of the produced polymers are still unclear [25, 34]. Therefore,the reaction mechanism of azo group incorporated polymerization is unclear. Until now,no detailed mass spectrometric characterization of azo group incorporated polymer has been reported. Herein,MALDI-TOF MS analysis is used as an effective method in our study to examine the detailed structure of azo group incorporated copolymer and the reaction mechanism of the copolymerization.
In this article,the copolymerization of EDA with CEC without catalyst by using EDA as the precursor of CEC is described. The copolymer is investigated based on MALDI-TOF MS analysis,from which comprehensive information including fragmentation and reaction mechanism is obtained. The results show that CEC was generated by nitrogen-elimination of EDA under microwave irradiation,and then it reacted with activated EDA to form CECco-EDA segments (Scheme 1). The same results can be found in the polymerization of EDA by enzyme-assisted system. Under microwave irradiation,higher Mn azo-incorporated polymers were obtained comparing with the metal-mediated methods [23, 24].

|
Download:
|
| Scheme 1. Nitrogen-elimination and copolymerization of EDA with carbethoxycarbene. | |
1H NMR spectra were recorded on a Mecury VX-300 spectrometer (300 MHz) using CDCl3 as solvent and TMS as the internal standard. Mn and polydispersity (Mw/Mn,PDI)oftheobtainedpolymers were determined by gel permeation chromatography (GPC) using polystyrene as standards with THF as eluent (1.0 mL/min). The GPC system was equipped with a Waters 717 plus auto sampler,a Waters 1515 isocratic HPLC pump,a Waters 2414 refractive index detector, and Shodex K-805,K-804,and K-802.5 columns in series. The temperatures of the columns and detector were both 30°C . The data of element analysis was determined by Varian-EL. Differential scanning calorimetry (DSC) was performed on a Q20 (TA) instrument with nitrogen as the protecting gas. The samples were heated from -30°C to 60°C ,held for 10 min to erase the thermal history,cooled to -30°C atarateof10°C/min,and finally heated to 60°C atarateof 10°C /min. X-ray diffraction (XRD) was recorded on a Bruker D8-advance with the scan speed of 6°C /min. Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectra were recorded on a Thermo iS10 spectrometer. Matrixassisted laser desorption ionization time-of-flight mass (MALDITOF MS) spectrum was collected with an Axima TOF2 mass spectrometry (Shimadzu). The instrument was equipped with a 3 ns pulse width 337 nm nitrogen laser. Sample solutions were deposited on the stainless steel target probe using the dried droplet method: 1 mL sample solution was dropped onto the target,and then 1 mL of CHCA matrix solution (2 mg/mL and 0.1% TFA in 50% ACN/H2O solution) was added. All of the mass spectra were obtained in positive ion mode at an accelerating voltage of 20 kV and 200 laser shots were averaged to generate each spectrum. Raman microspectroscopy spectra (excitation wavelength was 541.5 nm) were performed on an RM-1000 (Renishaw). Steady-state fluorescence emission spectra were recorded on an RF-5301PC (Shimadzu) with excitation wavelength of 521 nm and emission wavelength in the range of 500- 700 nm. For excitation spectra,the emission wavelength was 574 nm. The nitrogen-elimination and polymerization of EDA was carried out in a multimode microwave oven (SINEO-MAS-II, 50 Hz,1360 W) equipped with an infrared thermometric indicator. Thermo gravimetric analysis (TGA) curves were measured with a NETZSCH STA 449C thermal analyzer (NETZSCH,Germany). A few milligram of polymer was heated with a rate of 20°C /min from 50°C to 800°C under nitrogen atmosphere. Materials: ethyl diazoacetate was prepared according to literature [35]. Solvents and other materials were purchased from Sinopharm Chemical Reagent Co.,Ltd. The solvents were refluxed with calcium hydroxide for 24 h and distilled before used. Experimental Procedure of the polymerization of EDA by heating: Under argon atmosphere,0.02 mol EDA in a three-neck bottle was heated to 120°C . After the reaction was conducted for 2 h,the reaction mixture was characterized by 1H NMR and then proved that no polymerized product was obtained.
Experimental Procedure of the copolymerization of EDA with CEC under microwave irradiation: Under argon atmosphere, 0.02 mol EDA in a three-neck bottle (with or without N-435) without solvent was irradiated by microwaves for a predetermined time. The reaction mixture was then dissolved in acetone and precipitated by petrol ether. After filtration and vacuum drying,the samples were measured by GPC. No precipitated product can be separated from the acetone solution of the reaction mixture by petrol ether under conventional heating conditions (120°C ). 3. Results and discussion
Fig. 1 shows the temperature-time profiles of the reaction mixture. At the power level of 100 W,the temperature of the reaction mixture (without N-435) was about 50°C after 60 min and no polymerized product was obtained (Fig. 1a and c). The result indicates that under 100 W microwave irradiation,the activity of EDA is relatively low and no polymer is obtained. The activity of EDA is improved by N-435 under 100 W microwave irradiation (Fig. 1a) and an exothermic peak referring to the polymerization reaction can be observed [36, 37]. The result clearly indicates that enzyme (N-435) is a catalyst in the polymerization. Moreover,under 100 W microwave irradiation of EDA without N-435,no polymerized product was obtained. Fig. 1b is the temperature-time profiles of the reaction mixture with varied amounts of EDA at 200 W. For larger EDA amount,it took less time for the reaction mixture to reach the highest reaction temperature. After that,the temperature was kept constant near 110°C . When the power level increased to 200 W or 300 W,an exothermic peak connoting the occurrence of the polymerization can be observed in the temperature-time profiles (Fig. 1c) [38]. Moreover,it took less time for the reaction to achieve the highest reaction temperature under higher power of microwave irradiation.

|
Download:
|
| Fig. 1. Temperature-time profiles of the reaction mixture. (a) 0.02 mol EDA with different power level and different reaction system. (b) At 200 W with different amount of EDA (without enzyme). (c) At different power level with 0.02 mol EDA (without enzyme)). | |
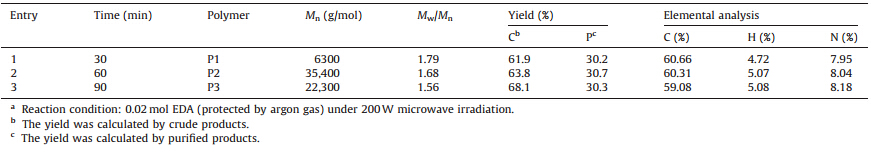
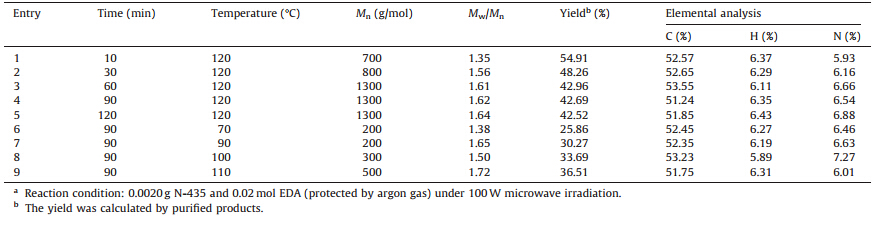
Table 1 lists the results of the polymerization under microwave irradiation at 200 W for 30,60,and 90 min,respectively. GPC curves of the obtained polymers that reprecipitated with petrol ther are listed in Fig. S1 (Supporting information). Polymerized products with Mn of 6300-35,400 g/mol were obtained,which contain nearly 8% of nitrogen by element analysis (Table 1). TheMn of the polymer after 90 min of the reaction time is lower than that obtained after 60 min,which may be due to the partial decomposition of the obtained polymer. Compared with the oligomers obtained by Pd or Al complexes,this method offers a quick and convenient method for the synthesis of polymer with higher Mn [23, 24]. Table 2 lists the results of enzyme-assisted nitrogen-elimination and copolymerization of CEC with EDA under 100 W microwave irradiation. Polymerized products withMnof 200-1300 g/mol were obtained,which contained nearly 6%-7% of nitrogen by element analysis. Tables 1 and 2 describe that the copolymerization of EDA with CEC without N-435 produces copolymer with higher molecule weight. However,the copolymerization of EDA with CEC needs lower microwave power and produces copolymers with higher yields. Moreover,results from Tables 1 and 2,as well as Fig. 1,show that N-435 decreased the energy barrier of the generation of CEC under 100 W microwave irradiation. However,polymers with lower Mn were produced because the activity of EDA decreased simultaneously.
| Table 1 Results of nitrogen-elimination and copolymerization of CEC with EDA under microwave irradiation.a |
| Table 2 Results of enzyme-assisted nitrogen-elimination and copolymerization of CEC with EDA under microwave irradiation.a |
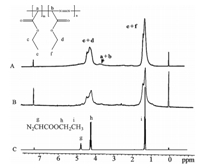
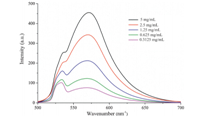
Fig. 2 shows the 1H NMR spectra of the purified products, exhibiting signals belonging to CH2 (4.0-4.6 ppm),CH (3.5- 3.8 ppm),and CH3 (1.0-1.6 ppm) groups on the polymer main chain [23]. The hydrogen resonance ascribed to CHN2group (from EDA) at 4.7 ppm disappeared after the polymerization. ATR-FTIR spectra of polymerized products are shown in Fig. 3,broad bands near 3000 cm-1 are due to CH,CH2,andCH3stretches of the aliphatic groups. The intense bands at 1720 cm-1 belong to the carbonyl bonds. The bands near the 1550 cm-1 assigned to the azo group are further confirmed by the Raman spectrum (Fig. S2 in Supporting information),showing the particular adsorption of azo group at 1550-1700 cm-1 [39]. The bands from 1370 cm-1 to 1020 cm-1 correspond to the absorbance of ester bonds. These results demonstrate the incorporation of azo group in the polymer. Fig. 4 presents the fluorescence spectra of the obtained copolymers synthesized by microwaves. The peaks at 570 nm referred to the azo groups,which are strong chromogenic groups on the polymer main chains [40]. Moreover,the intensity of the peak gradually decreases along with the decrease of the polymer concentration.
|
Download:
|
| Fig. 2. 1H NMR spectra of the polymerized product. (A) Enzyme-assisted under microwave irradiation; (B) microwave irradiation; (C) ethyl diazoacetate. | |

|
Download:
|
| Fig. 3. ATR-FTIR spectra of the polymerized product. Left,(a) enzyme-assisted under microwave irradiation; (b) microwave irradition) and FTIR spectrum of EDA (right). | |
|
Download:
|
| Fig. 4. Steady-state fluorescence emission spectra of the obtained copolymers synthesized by microwaves with different concentrations (in CHCl3). | |
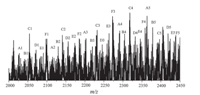
The accurate polymer structure is further confirmed by MALDITOF MS (Fig. S3 in Supporting Information). Fig. 5 is the MALDI-TOF MS spectrum of the obtained polymer which was synthesized under 200 W microwave irradiation withm/z from 2000 to 2450. In the MALDI-TOF MS spectrum,m/z peaks of homopolymerization of CEC or EDA cannot be observed,which proves the copolymerization product is poly(CEC-co-EDA). Regular m/z 86 and m/z 114 repeating patterns are recorded,which indicates the repeating units in the polymer main chain are -CH(COOEt)-(m/z 86) and - N==N-CH(COOEt)-(m/z 114). The mainm/z peaks in the range of 2020 to 2450 can be sorted as series Na+ + poly(CEC-co-EDA) and series K+ + poly(CEC-co-EDA) and match quite well with the two mass formulae below: For series Na+ + poly(CEC-co-EDA):
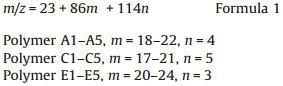
For series K+ + poly(CEC-co-EDA):
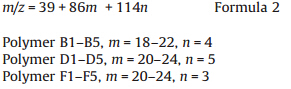
|
Download:
|
| Fig. 5. MALDI-TOF MS spectrum of poly(CEC-co-EDA) withm/zfrom 2000-2450 (P2). | |
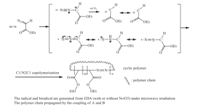
where 23 and 39 in the formula are them/z of Na+ and K+,and m and n are the number of -CH(COOEt)- and -N==N-CH(COOEt)-repeating unit,respectively. The data of MALDI-TOF MS for the two series are summarized in Tables S1 and S2 (Supporting information). Them/z of the end group is zero in the mass spectrum,which indicates that the obtained polymer is cyclic. These results clearly demonstrate that CH(COOEt) and biradical N==N-CH(COOEt) were generated from EDA by microwaves. The ratio of CEC and EDA in the copolymer is in a random pattern. For example,the number of CEC is in the range of 18 to 22 every four EDA units,while the number of EDA ranges from 3 to 5 every 20 CEC units in subseries A1-A5. This result is in agreement with that of the elemental analysis that shows 8% nitrogen is incorporated in the polymer. On the basis of the MALDI-TOF MS spectrum,the mechanism for the formation of poly(CEC-co-EDA) is shown in Scheme 2. First,CEC is generated from EDA under microwave irradiation (with or without N-435). Since the generation rate of CEC is faster than its homopolymerization reaction,N==N-CH(COOEt) biradicals are produced by the activation of CHN2 group in EDA by the unpolymerized CEC. Then the polymer chain propagates by the coupling of CH(COOEt) and N==N-CH(COOEt) and finally results in poly(CEC-co-EDA) copolymer. The polymerization reaction is thus termed as C1/N2C1 copolymerization. This proposed mechanism will be investigated by online FTIR or ESR (EPR) in the future. The incorporation of CEC and EDA in the polymer chain is random, which is suggested by the data of MALDI-TOF MS. Moreover, poly(CEC-co-EDA) is an amorphous polymer because of random incorporation of CEC and EDA fragments in the polymer chain [19]. As shown in Fig. S4 (Supporting information),no sharp peak referring to the crystallized peak of poly(CEC-co-EDA) is observed in the XRD curves. Moreover,the DSC curves (Fig. S5 in Supporting information) show Tg of the obtained polymers distributing in the range of -12.8 to -2.4°C ,which indicates different material properties (e.g. liquid crystalline) compared with the high Mn polymers obtained from Rh-catalysts without azo incorporation [41, 42]. TGA spectrum of the obtained polymer (Table 1,entry 2) is shown in Fig. S6 (Supporting information). The copolymer presents good thermal-stability,losing nearly 5% of its weight at 250°C .
|
Download:
|
| Scheme 2. Proposed mechanism for the nitrogen-elimination and copolymerization of EDA under microwave irradiation. | |
In conclusion,the metal-catalyst-free copolymerization of CEC and EDA proceeded successfully under microwave irradiation. Amorphous poly(CEC-co-EDA) with high Mn and azo group incorporation was obtained. By MALDI-TOF MS,it is elucidated that the polymer chain propagates by the co-coupling of CH(COOEt) and 1,3-azo-carbon biradical N==N-CH(COOEt)) to form poly(CEC-co-EDA) with more CEC units. Microwaves and lipase enzyme (N-435) provide an efficient,powerful and green approach for C1/N2C1 copolymerization.
AcknowledgmentThis project was supported by the National Natural Science Foundation of China (Nos. 21074097 and 21274112).
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.09.009.
| [1] | L. Xiao, L. Liao, X. Guo, L. Liu, One-pot synthesis of polyester-polyolefin copolymers by combining ring-opening polymerization and carbene polymerization, Macromol. Chem. Phys. 214 (2013) 2500-2506. |
| [2] | L.Q. Xiao, S.J. Cai, Q.Y. Liu, et al., One-step synthesis of polypyrazoles and selfassembled polypyrazole-copper catalysts for click chemistry, Polym. Chem. 5 (2014) 607-613. |
| [3] | Q. Lin, P. Chen, Y.P. Fu, et al., A green synthesis of a simple chemosensor that could instantly detect cyanide with high selectivity in aqueous solution, Chin. Chem. Lett. 24 (2013) 699-702. |
| [4] | S.J. Cai, L.Q. Xiao, L.Q. Liao, L.J. Liu, Catalyst-free cyclopropanation of alkenes with diazocompounds under microwave irradiation, Chin. J. Org. Chem. 33 (2013) 2602-2606. |
| [5] | L.Q. Xiao, Y. Li, X.X. Jia, L.Q. Liao, L.J. Liu, Microwave-assisted one-pot copolymerization of cyclic monomers and ethyl diazoacetate, Polym. Int. 63 (2014) 1154-1158. |
| [6] | L.Q. Xiao, L.Q. Liao, L.J. Liu, Chemical modification of graphene oxide with carbethoxycarbene under microwave irradiation, Chem. Phys. Lett. 556 (2013) 376-379. |
| [7] | B. Mohammadi, M. Adib, Microwave assisted one-pot tandem three-component synthesis of 2,4,5-triary1-2-4-dihydro-3H-1,2,4-triazol-3-one derivatives, Chin. Chem. Lett. 25 (2014) 553-556. |
| [8] | X.P. Ouyang, C.L. Liu, Y.X. Pang, X.Q. Qiu, Synthesis of a trimeric lignin model compound composed of a-O-4 and b-O-4 linkages under microwave irradiation, Chin. Chem. Lett. 24 (2013) 1091-1094. |
| [9] | L.J. Liu, S.J. Cai, Y. Tan, et al., Ring opening insertion polymerization of e-caprolactone with hydrogen phosphonate initiators, J. Polym. Sci. Part A: Polym. Chem. 47 (2009) 6214-6222. |
| [10] | S. Kobayashi, H. Uyama, S. Kimura, Enzymatic polymerization, Chem. Rev. 101 (2001) 3793-3818. |
| [11] | Y. Yanagishita, M. Kato, K. Toshima, S. Matsumura, Chemoenzymatic synthesis and chemical recycling of sustainable polyurethanes, ChemSusChem 1 (2008) 133-142. |
| [12] | L. Ragupathy, U. Ziener, R. Dyllick-Brenzinger, B. von Vacano, K. Landfester, Enzyme-catalyzed polymerizations at higher temperatures: synthetic methods to produce polyamides and new poly(amide-co-ester)s, J. Mol. Catal. B: Enzym. 76 (2012) 94-105. |
| [13] | F. He, S. Li, H. Garreau, M. Vert, R. Zhuo, Enzyme-catalyzed polymerization and degradation of copolyesters of ε-caprolactone and γ-butyrolactone, Polymer 46 (2005) 12682-12688. |
| [14] | F. He, S. Li, M. Vert, R. Zhuo, Enzyme-catalyzed polymerization and degradation of copolymers prepared from ε-caprolactone and poly(ethylene glycol), Polymer 44 (2003) 5145-5151. |
| [15] | A.J. Arduengo, R.L. Harlow, M. Kline, A stable crystalline carbene, J. Am. Chem. Soc. 113 (1991) 361-363. |
| [16] | C.M. Crudden, D.P. Allen, Stability and reactivity of N-heterocyclic carbene complexes, Coord. Chem. Rev. 248 (2004) 2247-2273. |
| [17] | M.K. Samantaray, V. Katiyar, D. Roy, et al., A cationic (N-heterocyclic carbene)-silver complex as catalyst for bulk ring-opening polymerization of L-lactides, Eur. J. Inorg. Chem. 2006 (2006) 2975-2984. |
| [18] | L. Xiao, Y. Li, L. Liao, L. Liu, Denitrogen alkene polymerization of bisdiazo compounds by copper(Ⅱ) catalysts, New J. Chem. 37 (2013) 1874-1877. |
| [19] | A.F. Noels, Carbene chemistry: stereoregular polymers from diazo compounds, Angew. Chem. 46 (2007) 1208-1210. |
| [20] | L. Liu, Y. Song, H. Li, Carbene polymerization: characterization of poly(carballyloxycarbene), Polym. Int. 51 (2002) 1047-1049. |
| [21] | E. Jellema, A.L. Jongerius, J.N. Reek, B. de Bruin, C1 polymerisation and related C-C bond forming ‘carbene insertion' reactions, Chem. Soc. Rev. 39 (2010) 1706-1723. |
| [22] | A.J.C. Walters, O. Troeppner, I. Ivanovic-Burmazovic, et al., Stereospecific carbene polymerization with oxygenated Rh(diene) species, Angew. Chem. Int. Ed. 51 (2012) 5157-5161. |
| [23] | E. Ihara, M. Kida, M. Fujioka, et al., Palladium-mediated copolymerization of diazocarbonyl compounds with phenyldiazomethane, J. Polym. Sci. Part A: Polym. Chem. 45 (2007) 1536-1545. |
| [24] | E. Ihara, M. Kida, T. Itoh, K. Inoue, Organoaluminum-mediated polymerization of diazoketones, J. Polym. Sci. Part A: Polym. Chem. 45 (2007) 5209-5214. |
| [25] | E. Ihara, K. Saiki, Y. Goto, T. Itoh, K. Inoue, Polycondensation of bis(diazocarbonyl) compounds with aromatic diols and cyclic ethers: synthesis of new type of polyetherketones, Macromolecules 43 (2010) 4589-4598. |
| [26] | E. Ihara, Y. Ishiguro, N. Yoshida, et al., (N-Heterocyclic carbene)Pd/borate initiating systems for polymerization of ethyl diazoacetate, Macromolecules 42 (2009) 8608-8610. |
| [27] | D.G.H. Hetterscheid, C. Hendriksen, W.I. Dzik, et al., Rhodium-mediated stereoselective polymerization of “carbenes”, J. Am. Chem. Soc. 128 (2006) 9746-9752. |
| [28] | E. Ihara, T. Hiraren, T. Itoh, K. Inoue, Palladium-mediated polymerization of cyclic diazoketones, J. Polym. Sci. Part A: Polym. Chem. 46 (2008) 1638-1648. |
| [29] | J.D. Clark, J.D. Heise, A.S. Shah, et al., Process research, development, and pilotplant preparation of clofencet, a novel wheat hybridizing agent: lewis acidcatalyzed reaction of ethyl diazoacetate with 4-chlorophenyl hydrazonoacetaldehyde, Org. Process Res. Dev. 8 (2004) 176-185. |
| [30] | O. Illa, C. Rodríguez-García, C. Acosta-Silva, et al., Cyclopropanation of cyclohexenone by diazomethane catalyzed by palladium diacetate: evidence for the formation of palladium(0) nanoparticles, Organometallics 26 (2007) 3306-3314. |
| [31] | E. Ihara, A. Nakada, T. Itoh, K. Inoue, Transition metal-mediated copolymerization of diazocarbonyl compounds with alkyne and isocyanide, Macromolecules 39 (2006) 6440-6444. |
| [32] | G.W. Cowell, A. Ledwith, Developments in the chemistry of diazo-alkanes, Quart. Rev. Chem. Soc. 24 (1970) 119-167. |
| [33] | N.M. Franssen, J.N. Reek, B. de Bruin, A different route to functional polyolefins: olefin-carbene copolymerization, Dalton Trans. 42 (2013) 9058-9068. |
| [34] | E. Ihara, H. Nishida, M. Fujii, T. Itoh, K. Inoue, Thermally induced polymerization and copolymerization with styrene of diazoketones in the presence of benzoquinone, Polym. Bull. 66 (2011) 3-15. |
| [35] | H.Z. Qi, Z.H. Yang, J.X. Xu, Synthesis of 3-alkoxy/aryloxy-beta-lactams using diazoacetate esters as ketene precursors under photoirradiation, Synthesis 5 (2011) 723-730. |
| [36] | L.Q. Liao, L.J. Liu, C. Zhang, F. He, R.X. Zhuo, Heating characteristics and polymerization of epsilon-caprolactone under microwave irradiation, J. Appl. Polym. Sci. 90 (2003) 2657-2664. |
| [37] | L.Q. Liao, L.J. Liu, C. Zhang, et al., Microwave-assisted ring-opening polymerization of epsilon-caprolactone, J. Polym. Sci. Part A: Polym. Chem. 40 (2002) 1749-1755. |
| [38] | L. Liao, L. Liu, C. Zhang, S. Gong, Microwave-assisted ring-opening polymerization of epsilon-caprolactone in the presence of ionic liquid, Macromol. Rapid Commun. 27 (2006) 2060-2064. |
| [39] | K. Maruoka, M. Oishi, H. Yamamoto, Novel anionic oligomerization by a new, sequential generation of organolithium compounds, Macromolecules 29 (1996) 3328-3329. |
| [40] | W.M. Nau, G. Greiner, H. Rau, et al., Fluorescence of 2,3-diazabicyclo[2.2.2]oct-2-ene revisited: solvent-induced quenching of the n,π*-excited state by an aborted hydrogen atom transfer, J. Phys. Chem. A 103 (1999) 1579-1584. |
| [41] | E. Jellema, A.L. Jongerius, A.J.C. Walters, et al., Ligand design in Rh(diene)-mediated “carbene” polymerization; efficient synthesis of high-mass, highly stereoregular, and fully functionalized carbon-chain polymers, Organometallics 29 (2010) 2823-2826. |
| [42] | E. Jellema, A.L. Jongerius, G.A. van Ekenstein, et al., Rhodium-mediated stereospecific carbene polymerization: from homopolymers to random and block copolymers, Macromolecules 43 (2010) 8892-8903. |