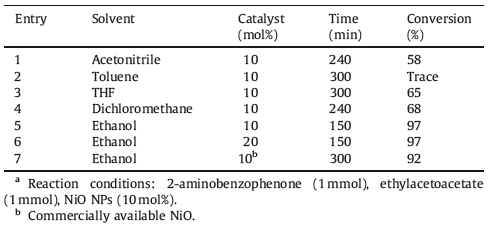
Quinolines are an important class of heterocycles due to their wide range of pharmacological properties such as antimalarial, anti-inflammatory,anti-asthmatic,antibacterial,antihypertensive, tyro-kinase PDGF-RTK inhibitory activities. The derivatives of quinolines are also used as synthetic building blocks in the synthesis of heterocycles [1, 2, 3, 4, 5, 6]. The classical methods to synthesize quinolines require harsh reaction conditions and the yields are unsatisfactory in most cases [7]. The Friedlander annulation,which involves the condensation of an aromatic 2-amino aldehyde/ ketone with ketone/aldehyde containing active methylene group followed by cyclization,is the simplest and most straightforward synthetic method for the synthesis of quinoline derivatives, especially for the highly substituted 3-functionalized quinolines [8]. Hydrochloric acid,sulfuric acid,p-toluenesulfonic acid and PPA have been employed as catalysts for this conversion [9, 10, 11, 12]. Some of the methods reported for the synthesis of quinolines also involve the use of catalysts such as SnCl2,BiCl3,I2 [12, 13],montmorillonite KSF clay [14],ionic liquids [15],Bi(OTf)3 [16],Y(OTf)3 [17],silver dodecatungstophosphate [18],silica sulfuric acid,sulfamic acid, neodymium nitrate [19, 20],dodecylphosphonic acid (DPA) [21], proline [22],silica supported phosphomolybdic acid [23],and sodium ethoxide [24]. However,most of these methods suffered from certain drawbacks such as harsh reaction conditions,use of expensive catalysts,and long reaction time,etc. Thus,a simple and environmentally benign procedure with an inexpensive and readily available reagent is desirable,which prompted us to test the feasibility of NiO nanoparticles (NiO NPs) as a catalyst for the synthesis of quinolines without any co-catalysts or additives. Hence in continuation of our earlier effort on quinolines [25, 26, 27, 28, 29, 30, 31, 32, 33] syntheses,herein we report an efficient,cost effective and environmentally benign procedure for the synthesis of multisubstituted quinolines (Scheme 1).
|
Download:
|
| Scheme 1.Synthesis of poly-substituted quinolones. | |
Chemicals were purchased from Sigma Aldrich,Fluka and used as received. IR spectra were recorded using an AVATAR 330 equipped with a DTGS detector. NMR spectra were recorded on a Bruker AMX-400 instrument in CDCl3 using TMS as an internal reference. Mass spectra were recorded either on an ABI QSTAR XL ESI-TOF Mass spectrometer (Sciex Model) or an LC-MS using Agilent 1200 series LC and Micromass zQ spectrometer. Melting points were determined in open capillaries and were uncorrected. Powder X-ray diffraction analysis was carried out using a Bruker D8 Advance diffractometer and lanthanum hexaboride (LaB6) was used to calibrate the instrument before analysis. 2.2. Synthesis of the catalyst
To a solution of 2.3 g of Ni(NO3)2⋅6H2O in 10 mL distilled water, 10 mL of 15% NaOH was added drop wise with constant stirring.After 15 min the obtained products were collected by centrifugation, washed thoroughly with distilled water and dried at 100 ℃. Then the dried sample was heated at 400 ℃ for 3 h.
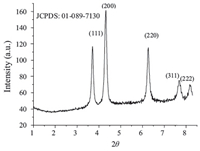
The synthesized NiO NPs were characterized using an XRD Bruker D8 Advance Diffractometer (Bruker AXS,Germany) with Cu Ka radiation (λ = 1.54Å ). The XRD pattern of NiO NPs powder samples was recorded over a 2θ range of 10-90°,which revealed that the synthesized NiO NPs were crystalline (Fig. 1). The XRD pattern showed four distinct diffraction peaks at 37.05,43.19, 62.67,75.12 and 79.02,which could be assigned to (1 1 1),(2 0 0), (2 2 0),(3 1 1) and (2 2 2) of cubic NiO NPs,respectively,and were in agreement with the database of Joint Committee on Powder Diffraction Standards (JCPDS No. 01-089-7130).
|
Download:
|
| Fig. 1.XRD pattern of synthesized NiO nanoparticles. | |
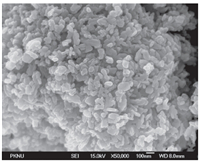
To determine the morphologies,the image of NiO NPs was taken using a scanning electron microscope (SEM) (Carl Zeiss Oxford Instrument) at various magnifications. The SEM micrographs (Fig. 2.) at lower magnification clearly revealed that the formation of well dispersed rod shaped nickel oxide nanostructures. SEM demonstrates that all the particles were in the size range of less than 100 nm.
|
Download:
|
| Fig. 2.SEM micrograph of synthesized NiO nanoparticles. | |
To a mixture of 2-aminoaryl ketone (1 mmol) and α-methylene carbonyl compound (1 mmol) in ethanol (5 mL) was added NiO NPs (10 mol%) and the mixture was refluxed. The progress of the reaction was monitored by TLC analysis. After the completion of the reaction,the reaction mixture was dissolved in ethanol to recover the catalyst by filtration and solvent was removed under reduced pressure. The obtained products were recrystallized using chloroform,characterized by IR,1H NMR,13C NMR and mass spectral data. The melting points and spectral data for the known compound are found to be identical to the values reported in the literature and for newly reported compounds the data are given below. 3. Results and discussion
By considering the advantageous surface characteristics of NiO NPs,we decided to investigate the behavior of different 2- aminoaryl ketones with various simple ketones,cyclic ketones and β-keto esters in the presence of NiO NPs as a catalyst. The compound 3a has been taken as a representative example and it was synthesized by treating 2-aminobenzophenone with ethyl acetoacetate in the presence NiO NPs as a catalyst in various solvents. Ethanol served as a better solvent than other solvents such as toluene,acetonitrile,dichloromethane and THF. After finding the suitable solvent the concentration of the catalyst was varied and optimized as 10 mol%. The yields of the products were found to be lower at lower amount than the optimized amount of catalyst and it remains unaffected above the optimized amount of the catalyst and reaction duration. The best results were obtained with10 mol% of the catalyst in ethanol (Table 1).
| Table 1 Optimization the reaction conditions under various solvents and various concentrations of the NiONPs catalyst for the representative compound 3a.a |
However,in the absence of NiO NPs,the reaction did not proceed even after long duration (10-12 h). Unlike other methods, the present procedure does not require either strong acids or high temperature to afford quinoline derivatives. This method has several advantages such as shorter reaction time with higher yields,cleaner product profiles,and simpler experimental and workup procedures. The reaction was carried out with different carbonyl compounds and 2-aminoaryl ketones to establish the generality and scope of the method. The obtained products were recrystallized using chloroform,characterized by IR,1H NMR,13C NMR and mass spectral data. All the spectral data are given as Supporting information. It was observed that the reactions of 2- amino benzophenone,2-amino-5-chlorobenzophenone,2-amino- 2',5-dichlorobenzophenone and 2-amino-5-nitrobenzophenone with various carbonyl compounds including cyclic,acyclic β-diketones, aliphatic and aromatic β-diketones,ketones and β-diketones proceeded efficiently and the desired products were obtained in high yields in short reaction time. The reaction duration and the yields are given in Table 2. In addition,this method is equally effective for both cyclic and acyclic ketones. Moreover,in most of the cases,2-aminobenzophenone and 2-amino-5-chlorobenzophenone in comparison with 2-amino- 20,5-dichlorobenzophenone and 2-amino-5-nitrobenzophenone afforded the corresponding quinolines in shorter reaction time.
| Table 2 Nano-NiO2 catalyzed synthesis of polysubstituted quinolines.a |
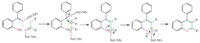
The NiO NPs indeed displayed better activity compared to the commercially available NiO since the NiO NPs have Lewis acid sites (Ni2+) and Lewis base sites (O2-). Thus,a Lewis base site (O2-) of NiO NPs deprotonate the a-methylene group,and the Lewis acid site (Ni2+) can activate the carbonyl group for the imine formation (Fig. 3).
|
Download:
|
| Fig. 3.Plausible mechanism. | |
The efficacy of the NiO NPs as a catalyst was compared with that of other reported catalysts such as Bi(OTf)3,Y(OTf)3,Ag3PW12O40, NaHSO4⋅SiO2 and KSF clay using 3a as a representative example and found to be equally efficient. The results given in Table 3 clearly reveal that the present protocol (using NiO NPs) could serve as a better method than the existing methods.
| Table 3 Comparison of the efficiency of NiO NPs for synthesis of quinoline 3a.a |
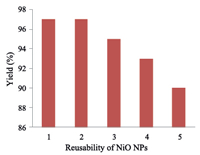
The NiO catalyst can be recovered easily,since it is insoluble in organic solvents. Furthermore,we investigated the reusability of the catalyst. After the completion of the reaction,the catalyst has been recovered by filtration,washed three times with 5 mL of methanol,dried at 100 ℃ overnight,and then subjected to a second run of the reaction with the same substrates. Fig. 4 shows that the yields of 3a in the second and third runs were almost identical to that in the first use. In every case,greater than 92% of the NiO NPs was easily recovered from the reaction mixture by filtration. In the fourth and fifth runs 93% yield of 3a was obtained. Fig. 5 revealed that even after the fifth run the morphology of the NiO NPs was not affected much.
|
Download:
|
| Fig. 4.Reusability of NiO NPs. | |

|
Download:
|
| Fig. 5.XRD pattern of used NiO nanoparticles (after the five runs). | |
In summary,a new method has been developed for the synthesis of poly-substituted quinolines under Friedla¨nder heteroannulation conditions using NiO NPs as a heterogeneous and reusable catalyst. The advantages of this method are the efficiency, generality,high yields with short reaction time,low cost,clean product profile,simplicity,ease of preparation of the catalyst,ease of product isolation,and compliance with the green chemistry protocols.
AcknowledgmentsThe authors are thankful to the VIT University for providing the generous support to carry out this work and also thankful to the SIF-Chemistry for providing the NMR,IR facilities and powder Xray diffraction studies.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.06.026.
| [1] | R.D. Larsen, E.G. Corley, A. King, et al., Practical route to a new class of LTD4 receptor antagonists, J. Org. Chem. 61 (1996) 3398-3405. |
| [2] | Y.L. Chen, K.C. Fang, J.Y. Sheu, S.L. Hsu, C.C.J. Tzeng, Synthesis and antibacterial evaluation of certain quinolone derivatives, Med. Chem. 44 (2001) 2374-2377. |
| [3] | D. Doubé, M. Blouin, C. Brideau, et al., Quinolines as potent 5-lipoxygenase inhibitors: synthesis and biological profile of L-746,530, Bioorg. Med. Chem. Lett. 8 (1998) 1255-1260. |
| [4] | M.P. Maguire, K.R. Sheets, K. McVety, A.P. Spada, A. Zilberstein, A new series of PDGF receptor tyrosine kinase inhibitors: 3-substituted quinoline derivatives, J. Med. Chem. 37 (1994) 2129-2137. |
| [5] | S. Asghari, S. Ramezani, M. Mohseni, Synthesis and antibacterial activity of ethyl 2-amino-6-methyl-5-oxo-4-aryl-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carboxylate, Chin. Chem. Lett. 25 (2014) 431-434. |
| [6] | M.B. Kanani, M.P. Patel, Synthesis of N-arylquinolone derivatives bearing 2-thiophenoxy quinolines and their antimicrobial evaluation, Chin. Chem. Lett. (2014), http://dx.doi.org/10.1016/j.cclet.2014.04.002. |
| [7] | S.E. Denmark, S. Venkatraman, On the mechanism of the Skraup-Doebner-Von miller quinoline synthesis, J. Org. Chem. 71 (2006) 1668-1676. |
| [8] | E.A. Fehnel, Friedländer syntheses with o-aminoaryl ketones. I. Acid-catalyzed condensations of o-aminobenzophenone with ketones, J. Org. Chem. 31 (1966) 2899-2902. |
| [9] | R.P. Thummel, The application of Friedländer and Fischer methodologies to the synthesis of organized polyaza cavities, Syn. Lett. (1992) 1-12. |
| [10] | S. Glaldiali, G. Chelucci, M.S. Mudadu, M.A. Gastaut, R.P. Thummel, Friedländer synthesis of chiral alkyl-substituted 1,10-phenanthrolines, J. Org. Chem. 66 (2001) 400-405. |
| [11] | L. Strekowski, A. Czarny, The Friedländer synthesis of 4-perfluoroalkylquinolines, J. Fluorine Chem. 104 (2000) 281-284. |
| [12] | G.W. Wang, C.S. Jia, Efficient solvent-free synthesis of quinolines promoted by BiCl3, Lett. Org. Chem. 3 (2006) 289-291. |
| [13] | J. Wu, H.G. Xia, K. Gao, Molecular iodine: a highly efficient catalyst in the synthesis of quinolines via Friedländer annulation, Org. Biomol. Chem. 4 (2006) 126-129. |
| [14] | J.S. Yadav, B.V.S. Reddy, V. Sunitha, K. Srinivasa Reddy, K.V.S. Ramakrishna, Montmorillonite KSF-catalyzed one-pot synthesis of hexahydro-1H-pyrrolo[3,-c]quinoline derivatives, Tetrahedron Lett. 45 (2004) 7947-7950. |
| [15] | S.S. Palimkar, S.A. Siddiqui, T. Daniel, R.J. Lahoti, K.V. Srinivasan, Ionic liquidpromoted regiospecific Friedlander annulation: novel synthesis of quinolines and fused polycyclic quinolines, J. Org. Chem. 68 (2003) 9371-9378. |
| [16] | J.S. Yadav, B.V.S. Reddy, K. Premalatha, Bi(OTf)3-catalyzed Friedländer heteroannulation: a rapid synthesis of 2,3,4-trisubstituted quinolines, Syn. Lett. (2004) 963-966. |
| [17] | S.K. De, R.A. Gibbs, A mild and efficient one-step synthesis of quinolines, Tetrahedron Lett. 46 (2005) 1647-1649. |
| [18] | J.S. Yadav, B.V.S. Reddy, P. Sreedhar, R. Srinivasa Rao, K. Nagaiah, Silver phosphotungstate: a novel and recyclable heteropoly acid for Friedländer quinoline synthesis, Synthesis (2004) 2381-2385. |
| [19] | J.S. Yadav, P. Purushottama Rao, D. Sreenu, et al., Sulfamic acid: an efficient, costeffective and recyclable solid acid catalyst for the Friedlander quinoline synthesis, Tetrahedron Lett. 46 (2005) 7249-7253. |
| [20] | R. Varala, R. Enugala, S.R. Adapa, Efficient and rapid Friedlander synthesis of functionalized quinolines catalyzed by neodymium(iii) nitrate hexahydrate, Synthesis (2006) 3825-3830. |
| [21] | S. Ghassamipour, A.R. Sardarian, Friedländer synthesis of poly-substituted quinolines in the presence of dodecylphosphonic acid (DPA) as a highly efficient, recyclable and novel catalyst in aqueous media and solvent-free conditions, Tetrahedron Lett. 50 (2009) 514-519. |
| [22] | B. Jiang, J. Dong, Y. Jin, X.L. Du, M. Xu, The first proline-catalyzed Friedlander annulation: regioselective synthesis of 2-substituted quinoline derivatives, Eur. J. Org. Chem. (2008) 2693-2696. |
| [23] | B. Das, M. Krishnaiah, K. Laxminarayana, D. Nandankumar, Silica supported phosphomolybdic acid: an efficient heterogeneous catalyst for Friedlander synthesis of quinolines, Chem. Pharm. Bull. 56 (2008) 1049-1051. |
| [24] | D. Yang, K. Jiang, J. Li, F. Xu, Synthesis and characterization of quinoline derivatives via the Friedländer reaction, Tetrahedron 63 (2007) 7654. |
| [25] | S. Sarveswari, V. Vijayakumar, An efficient microwave assisted eco-friendly synthesis of 6-chloro-3-(3-arylacryl oyl)-2-methyl-4-phenylquinolines and their conversion to 6-chloro-3-(1-phenyl-5-aryl-4,5-dihydro-1H-pyrazol-3-yl)-2-methyl-4-phenylquinolines, J. Chin. Chem. Soc. 59 (2012) 66-71. |
| [26] | W.S. Loh, H.K. Fun, S.V. Sarveswari, B.P. Vijayakumar, Reddy, 1-(6-Chloro-2-methyl-4-phenylquinolin-3-yl)-3-(3-methoxyphenyl)prop-2-en-1-one, Acta Crystallogr. E66 (2010) o91-o92. |
| [27] | W.S. Loh, H.K. Fun, S. Sarveswari, V. Vijayakumar, B.P. Reddy, 6-Chloro-3-[5-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-3-yl]-2-methyl-4-phenylquinoline, Acta Crystallogr. E66 (2010) o304. |
| [28] | W.S. Loh, H.K. Fun, S. Sarveswari, V. Vijayakumar, B.P. Reddy, (E)-1-(6-Chloro-2-methyl-4-phenyl-3-quinolyl)-3-(2-methoxyphenyl)prop-2-en-1-one, Acta Crystallogr. E66 (2010) o353-o354. |
| [29] | T. Shahani, H.K. Fun, S. Sarveswari, V. Vijayakumar, B.P. Reddy, 3-Acetyl-6-chloro-2-methyl-4-phenylquinolinium perchlorate, Acta Crystallogr. E66 (2010) o1192-o1193. |
| [30] | S. Natarajan, P. Indumathi, B.P. Reddy, V. Vijayakumar, P.L.N. Lakshman, Ethyl 2-methyl-5-oxo-4-(3,4,5-trimethoxyphenyl)-1,4,5,6,7,8-hexahydro quinoline-3-carboxylate, Acta Crystallogr. E66 (2010) o2240. |
| [31] | K. Rajesh, B.P. Reddy, V. Vijayakumar, Synthesis and biological evaluation of 4-(4-(di-(1H-indol-3-yl)methyl)phenoxy)-2-chloroquinolines, Indian J. Heterocycl. Chem. 19 (2009) 95-96. |
| [32] | S. Sarveswari, V. Vijayakumar, A rapid microwave assisted synthesis of 1-(6-Chloro-2-methyl-4-phenylquinolin-3-yl)-3-(aryl)prop-2-en-1-ones and its anti bacterial and anti fungal evaluation, Arabian J. Chem. (2011), http://dx.doi.org/10.1016/j.arabjc.2011.01.032 (in press). |
| [33] | S. Sarveswari, V. Vijayakumar, Synthesis and characterization of new 3-(4 5-dihydro-5-aryl)isoxazol-3-yl)-4-hydroxyl quinolin-2(1H)-ones and 3-(4-styryl)isoxazolo[4,5-c]quinolin-4(5H)-one derivatives, Arabian J. Chem. (2011), http:// dx.doi.org/10.1016/j.arabjc.2011.09.020 (in press). |