As it is very well known that pyridin-4-(1H)-ones are key structural elements in medicinal chemistry and versatile intermediates in organic synthesis [1]. Many derivatives have been studied as potential treatments for a range of diseases because of their important biological properties,such as antibacterial [2], antiplatelet [3],antitumor [4],and other pharmacological activities. On the other hand,thiophene derivatives are important heterocycles found in numerous biologically active and natural compounds [5]. The interest in this class of heterocycles has spread from dye chemistry [6] to modern drug design [7],and biodiagnostics [8]. The versatility of the Gewald reaction to prepare 2-aminothiophenes with a high degree of functionality is well known [9].
Azulene derivatives have attracted interest in medicine as antiulcer drugs [10],anticancer agents [11],and as antioxidant therapeutics for neurodegenerative conditions [12]. A variety of heterocycle-fused and substituted azulenes have so far been obtained by many research groups [13] based on the promising viewpoints of chemical properties and physiological activities [14].
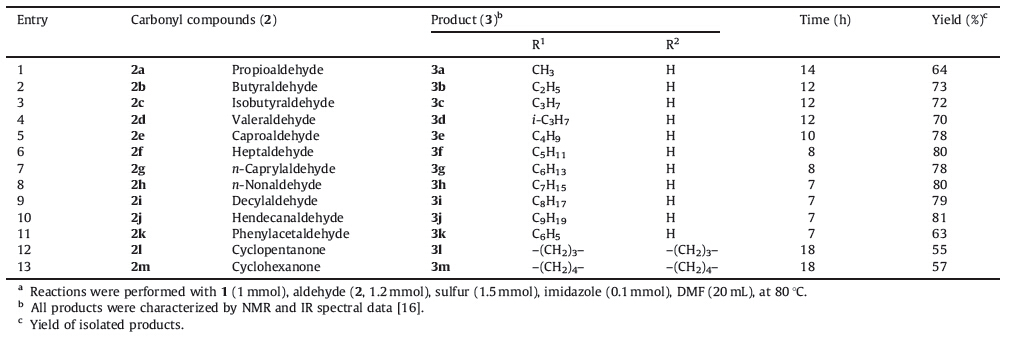
As part our work concerning the synthesis of heterocycle-fused azulenes with potential therapeutic interest,we recently reported an efficient synthesis of pyridinone-fused azulenes from ethyl 1- cyanoacetyl-2-methoxyazulene-3-carboxylate via a tandem addition- elimination-SNAr reaction [15]. Herein,we report on the synthesis of 11H(2H)-4-oxo-thiophene[3',4':6,5]pyrido[3,2-a]azulene- 10-carboxylates (3) via the Gewald reaction of ethyl 1- cyanoacetyl-2-methoxyazulene-3-carboxylate (1) with carbonyl compounds (2),and elemental sulfur in the presence of imidazole as catalyst (Scheme 1).
|
Download:
|
| Scheme 1.Syntheses of 4-oxothiophene[3',4':6,5]pyrido[3,2-a]azulenes. | |
A mixture of ethyl 1-cyanoacetyl-2-methoxyazulene-3-carboxylate (1) (1.0 mmol),carbonyl compounds (2) (1.2 mmol),sulfur (1.5 mmol) and imidazole (0.1 mmol) in DMF (20 mL) was stirred at 80 ℃ for 7-18 h. At the end of the reaction,50 mL of water was added and the mixture was extracted with EtOAc (10 mL × 3). The solution was dried with Na2SO4,filtered and concentrated with reduced pressure. The residue was purified by column chromatography on silica gel using n-hexane/EtOAc (3/1,v/v) as eluent to give the pure products (3a-m). 3. Results and discussion
In this study,we first optimized the reaction of ethyl 1-cyanoacetyl-2-methoxyazulene-3-carboxylate (1) with butyraldehyde (2b) and elemental sulfur as model substrates. In our preliminary experiments,1.0 mmol of 1 was treated with 1.2 mmol of 2b,with 1.5 mmol elemental sulfur and imidazole as catalysts in DMF at 80 ℃. The reaction was completed in 12 h. After work-up of the reaction mixture,ethyl 2-ethyl-11H(2H)-4- oxothiophene[3',4':6,5]pyrido[3,2-a]azulene-10-carboxylate (3b) was obtained in 73% yield as orange needles (mp 96-98 ℃). Its structure was determined from the spectral data as well as elemental analysis (C20H17NO3S). Moreover,the yield of 3b decreased in DMF to 54% and 67%,at 50 ℃ and 100 ℃,respectively.
In an initial endeavor,we carried out the reaction of ethyl 1- cyanoacetyl-2-methoxyazulene-3-carboxylate (1) with butyraldehyde (2b) and elemental sulfur using various catalysts in DMF at 80 ℃. The screening results of the reaction were summarized in Table 1. Classical Gewald reaction catalysts,such as diethylamine, morpholine L-proline,N-methylimidazole,and imidazole were tested to catalyze the multi-component condensation reaction. As shown in Table 1,the best yield was obtained when this reaction was conducted with 10 mol% imidazole as catalyst,and DMF as a solvent.
| Table 1 Effect of various catalyst on the model reaction.a |
Furthermore,we also tested different solvents,such as dichloromethane,1,2-dichloroethane,ethanol,acetonitrile,chloroform, tetrahydrofuran and toluene in this reaction,but the desired product 3b was obtained in lower yields.
Under the optimized conditions,a wide range of carbonyl compounds (2) underwent this one-pot condensation with ethyl 1-cyanoacetyl-2-methoxyazulene-3-carboxylate (1) to give the corresponding 11H(2H)-4-oxothiophene[3',4':6,5]pyrido[3,2- a]azulene-10-carboxylates (3). The results are summarized in Table 2. For alkyl aldehydes,the reaction proceeded smoothly in all cases (Table 2,entries 1-11).
| Table 2 Synthesis of 4-oxothiophene[3',4':6,5]pyrido[3,2-a]azulenes 3.a |
Next,some cyclic ketones,such as cyclopentanone and cyclohexanone,also proceeded well under the optimized conditions give the corresponding products 3l and 3m,in 55% and 57% yields (Table 2,entries 12 and 13),respectively.
The structural elucidation of the products was determined by IR,1H NMR,13C NMR spectral data and elemental analysis.
The proposed mechanism of the process is summarized in Scheme 2. The sequence involves an initial conjugate addition of the carbonyl compound (2) and elemental sulfur to 1 to give adduct E via Gewald reaction. This then undergoes the domino SNAr cyclizations of the 2-methoxy at the azulene moiety by attack of the NH2 group to yield the tetracyclic system 3.

|
Download:
|
| Scheme 2.A proposed mechanism for the formation of 3. | |
In summary,we have developed an efficient method to prepare a series of 4-oxothiophene[3',4':6,5]pyrido[3,2-a]azulenes via tandem Gewald and SNAr cyclization reactions in moderate to good yields. The mild conditions,operational simplicity and generality of the reaction should render this new domino reaction as useful for introducing great molecular diversity. Further investigations to elaborate on the scope of this methodology and to show the synthetic utility of the resulting heterocycle-fused azulene derivatives are currently in progress.
AcknowledgmentsWe are grateful for financial support from the Science and Technology Department of Liaoning Province (No. 2011220022).
| [1] | P.M. Weintraub, J.S. Sabol, J.M. Kane, D.R. Borcherding, Recent advances in the synthesis of piperidones and piperidines, Tetrahedron 59 (2003) 2953-2989. |
| [2] | D.D. Erol, N. Yulug, Synthesis and antimicrobial investigation of thiazolinoalkyl-4(H)-pyridiones, Eur. J. Med. Chem. 29 (1994) 893-897. |
| [3] | L.J. Huang, M.C. Hsieh, C.M. Teng, K.H. Lee, S.C. Kuo, Synthesis and antiplatelet activity of phenyl quinolones, Bioorg. Med. Chem. 6 (1998) 1657-1662. |
| [4] | C.T. Chen, M.H. Hsu, Y.Y. Cheng, et al., Synthesis and in vitro anticancer activity of 6,7-methylenedioxy (or 5-hydroxy-6-methoxy)-2-(substituted selenophenyl)-quinolin-4-one analogs, Eur. J. Med. Chem. 46 (2011) 6046-6056. |
| [5] | T.S. Jagodzinski, Thioamides as useful synthons in the synthesis of heterocycles, Chem. Rev. 103 (2003) 197-228. |
| [6] | M.S.Yen, I.J.Wang, Synthesis andabsorptionspectra ofhetarylazodyes derived from coupler 4-aryl-3-cyano-2-aminothiophenes, Dyes Pigments 61 (2004) 243-250. |
| [7] | C. Wu, E.R. Decker, N. Blok, et al., Discovery, modeling, and human pharmacokinetics of N-(2-acetyl-4,6-dimethylphenyl)-3-(3,4-dimethyl isoxazol-5-ylsulfamoyl) thiophene-2-carboxamide (TBC3711), a second generation, ETA selective, and orally bioavailable endothelin antagonist, J. Med. Chem. 47 (2004) 1969-1986. |
| [8] | K. Doré, S. Dubus, H.A. Ho, et al., Fluorescent polymeric transducer for the rapid, simple, and specific detection of nucleic acids at the zeptomole level, J. Am. Chem. Soc. 126 (2004) 4240-4244. |
| [9] | (a) K. Gewald, Heterocyclen aus CH-aciden nitrilen, VII. 2-Amino-thiophene aus a-oxo-mercaptanen und methylenaktiven nitrilen, Chem. Ber. 98 (1965) 3571-3577; (b) K. Gewald, M. Gruner, U. Hain, G. Sü ptitz, Zur ringumwandlung von 2-aminothiophen-3-carbonsäureestern: pyridon-und pyridazinon-derivate, Monatsh. Chem. 119 (1988) 985-992; (c) X.G. Huang, J. Liu, J. Ren, et al., A facile and practical one-pot synthesis of multisubstituted 2-aminothiophenes via imidazole-catalyzed Gewald reaction, Tetrahedron 67 (2011) 6202-6205. |
| [10] | T. Yanagisawa, S. Wakabayashi, T. Tomiyama, et al., Synthesis and anti-ulcer activities of sodium alkylazulene sulfonates, Chem. Pharm. Bull. 36 (1988) 641-647. |
| [11] | (a) A.E. Asato, A. Peng, M.Z. Hossain, et al., Azulenic retinoids: novel nonbenzenoid aromatic retinoids with anticancer activity, J. Med. Chem. 36 (1993) 3137-3147; (b) B.C. Hong, Y.F. Jiang, E.S. Kumar, Microwave-assisted [6 + 4]-cycloaddition of fulvenes and α-pyrones to azulene-indoles: facile syntheses of novel antineoplastic agents, Bioorg. Med. Chem. Lett. 11 (2001) 1981-1984. |
| [12] | D.A. Becker, J.J. Ley, L. Echegoyen, et al., Stilbazulenyl nitrone (STAZN): a nitronylsubstituted hydrocarbon with the potency of classical phenolic chain-breaking antioxidants, J. Am. Chem. Soc. 124 (2002) 4678-4684. |
| [13] | (a) T. Morita, T. Nakadate, K. Takase, A facile method for the synthesis of azuleno[2,1-b]furan and azuleno[2,1-b]pyrrole derivatives and their some properties, Heterocycles 15 (1981) 835-838; (b) M. Nishiura, I. Ueda, K. Yamamura, Synthesis of 4-(azuleno[b]indolyl)-3-buten-2-ones by intramolecular tropylium ion-mediated furan ring-unravelled reaction, Heterocycles 74 (2007) 951-960; (c) S. Ito, T. Okujima, S. Kikuchi, et al., Synthesis and intramolecular pericyclization of 1-azulenyl thioketones, J. Org. Chem 73 (2008) 2256-2263; (d) D.L. Wang, S.F. Li, W. Li, et al., An efficient synthesis of 3-(guaiazulene-1-yl)succinimides by addition of guaiazulene to maleimides, Chin. Chem. Lett. 22 (2011) 789-792; (e) D.L. Wang, Z. Dong, J. Xu, D. Li, An efficient synthesis of 2-(guaiazulen-1-yl)furan derivatives via intramolecular Wittig reactions, Chin. Chem. Lett. 24 (2013) 622-624. |
| [14] | G. Fischer, Chapter 3. Azulenes fused to heterocycles, Advances in Heterocyclic Chemistry, vol. 97, 2009, pp. 131-238. |
| [15] | (a) D.L. Wang, Q.T. Cui, S.S. Feng, et al., A new synthesis approach to azuleno[2,1-b]pyridine-4(1H)-ones, Heterocyles 85 (2012) 697-704; (b) D.L. Wang, Z. Dong, Q.T. Cui, et al., Synthesis of some pyrazole-fused pyrido[3,2-a]azulenes, Heterocyles 87 (2013) 2343-2350. |
| [16] | Physical and spectral (IR, NMR, Anal.) data: 3a:Mp 110-112 ℃. IR (KBr, cm-1): ν 3423 (NH), 1674 (C5O), 1658 (C5O). 1H NMR (400 MHz, CDCl3): δ 1.58 (t, 3H, J = 7.2 Hz), 2.36 (s, 3H), 4.55 (q, 2H, J = 7.2 Hz), 7.54 (s, 1H), 7.80-7.87 (m, 3H), 9.46 (d, 1H, J = 10.4 Hz), 10.22 (d, 1H, J = 10.0 Hz), 11.32 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 16.1, 27.2, 60.4, 101.3, 112.3, 117.5, 127.8, 131.9, 133.7, 135.2, 135.8, 137.3, 137.6, 142.3, 144.4, 145.5, 150.7, 165.2, 172.5. Anal. Calcd. for C19H15NO3S: C 67.64, H 4.48, N 4.15, S 9.50; Found: C 67.79, H 4.64, N 4.27, S 9.61. 3b: Mp 96-98 ℃. IR (KBr, cm-1): ν 3414 (NH), 1684 (C5O), 1653 (C5O). 1H NMR (400 MHz, CDCl3): δ 1.37 (t, 6H, J = 7.2 Hz), 1.55 (t, 3H, J = 7.2 Hz), 2.88 (q, 2H, J = 7.2 Hz), 4.56 (q, 2H, J = 7.2 Hz), 7.51 (s, 1H), 7.81-7.90 (m, 3H), 9.43 (d, 1H, J = 10.4 Hz), 10.20 (d, 1H, J = 10.0 Hz), 11.36 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 14.6, 15.2, 25.6, 60.7, 100.2, 112.1, 118.6, 127.7, 132.6, 133.4, 135.7, 135.9, 137.3, 137.9, 142.0, 144.4, 145.7, 150.5, 165.5, 172.8. Anal. Calcd. for C20H17NO3S: C 68.36, H 4.88, N 3.99, S 9.12; Found: C 68.45, H 4.95, N 4.16, S 9.24. 3c: Mp 165-167 ℃. IR (KBr, cm-1): ν 3424 (NH), 1682 (C5O), 1658 (C5O). 1H NMR(400 MHz, CDCl3): δ 1.01 (t, 3H, J = 3.6 Hz), 1.53 (t, 3H, J = 7.2 Hz), 1.72-1.77 (m, 2H), 2.80-2.83 (m, 2H), 4.55 (q, 2H, J = 7.2 Hz), 7.41 (s, 1H), 7.78-7.81 (m, 1H), 7.85-7.88 (m, 2H), 9.41 (d, 1H, J = 10.0 Hz), 10.20 (d, 1H, J = 9.2 Hz), 11.53 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 13.6, 14.7, 24.2, 32.3, 60.6, 100.1, 112.2, 119.3, 127.8, 132.2, 133.3, 135.6, 136.1, 136.8, 137.1, 142.1, 144.2, 146.6, 150.6, 166.4, 173.5. Anal. Calcd. for C21H19NO3S: C 69.02, H 5.24, N 3.83, S 8.77; Found: C 69.19, H 5.34, N 3.95, S 8.89. 3d:Mp 174-176 ℃. IR (KBr, cm-1): ν 3435 (NH), 1689 (C5O), 1643 (C5O). 1H NMR (400 MHz, CDCl3): δ 1.38 (d, 6H, J = 6.8 Hz), 1.54 (t, 3H, J = 6.8 Hz), 3.17-3.20 (m, 1H), 4.56 (q, 2H, J = 6.8 Hz), 7.46 (s, 1H), 7.80-7.87 (m, 3H), 9.42 (d, 1H, J = 10.0 Hz), 10.21 (d, 1H, J = 10.0 Hz), 11.57 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 14.7, 34.4, 36.1, 60.6, 100.1, 112.3, 117.1, 127.8, 132.4, 133.3, 135.6, 135.8, 137.2, 142.8, 143.6, 144.2, 145.4, 150.6, 166.2, 173.3. Anal. Calcd. for C21H19NO3S: C 69.02, H 5.24, N 3.83, S 8.77; Found: C 69.13, H 5.36, N 3.98, S 8.84. 3e:Mp 138-139 ℃. IR (KBr, cm-1): ν 3483 (NH), 1692 (C5O), 1664 (C5O). 1H NMR (400 MHz, CDCl3): δ 0.94 (t, 3H, J = 3.6 Hz), 1.40-1.45 (m, 2H), 1.55 (t, 3H, J = 7.2 Hz), 1.67-1.73 (m, 2H), 2.83-2.87 (m, 2H), 4.56 (q, 2H, J = 7.2 Hz), 7.47 (s, 1H), 7.81-7.89 (m, 3H), 9.43 (d, 1H, J = 10.0 Hz), 10.22 (d, 1H, J = 10.4 Hz), 11.51 (s, 1H). 13CNMR(100 MHz, CDCl3): δ 13.8, 14.7, 22.0, 29.9, 32.2, 60.7, 100.1, 112.2, 119.2, 127.7, 132.5, 133.4, 135.5, 136.5, 136.9, 137.3, 142.1, 144.3, 146.7, 150.6, 166.4, 172.8. Anal. Calcd. for C22H21NO3S: C 69.63, H 5.58, N 3.69, S 8.45; Found: C 69.78, H 5.76, N 3.83, S 8.56. 3f:Mp 127-129 ℃. IR (KBr, cm-1): ν 3433 (NH), 1687 (C5O), 1659 (C5O). 1H NMR (400 MHz, CDCl3): δ 0.89 (t, 3H, J = 3.6 Hz), 1.36-1.38 (m, 4H), 1.53 (t, 3H, J = 6.8 Hz), 1.72-1.73 (m, 2H), 2.81-2.83 (m, 2H), 4.54 (q, 2H, J = 6.8 Hz), 7.39 (s, 1H), 7.80-7.87 (m, 3H), 9.40 (d, 1H, J = 10.0 Hz), 10.20 (d, 1H, J = 10.4 Hz), 11.54 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 14.0, 14.6, 22.4, 30.2, 30.8, 31.2, 60.7, 100.1, 112.2, 120.2, 127.8, 132.3, 133.2, 135.6, 136.4, 136.7, 137.1, 142.0, 144.1, 146.6, 150.5, 166.6, 173.3. Anal. Calcd. for C23H23NO3S: C 70.20, H 5.89, N 3.56, S, 8.15; Found: C 70.34, H 5.95, N 3.70, S, 8.29. 3g:Mp 125-127 ℃. IR (KBr, cm-1): ν 3493 (NH), 1695 (C5O), 1643 (C5O). 1H NMR (400 MHz, CDCl3): δ 0.88 (t, 3H, J = 3.6 Hz), 1.30-1.39 (m, 6H), 1.53 (t, 3H, J = 6.8 Hz), 1.69-1.73 (m, 2H), 2.81-2.85 (m, 2H), 4.55 (q, 2H, J = 6.8 Hz), 7.39 (s, 1H), 7.77-7.85 (m, 3H), 9.40 (d, 1H, J = 10.0 Hz), 10.19 (d, 1H, J = 10.4 Hz), 11.25 (s, 1H). 13CNMR(100 MHz, |