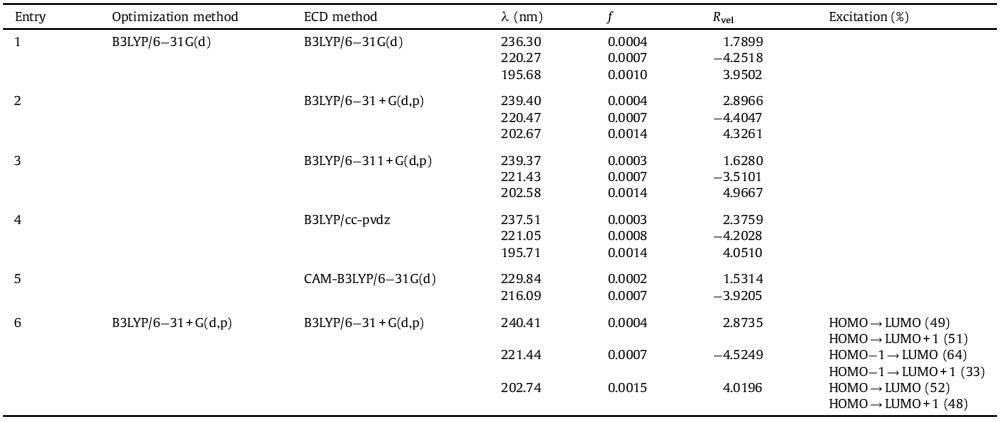
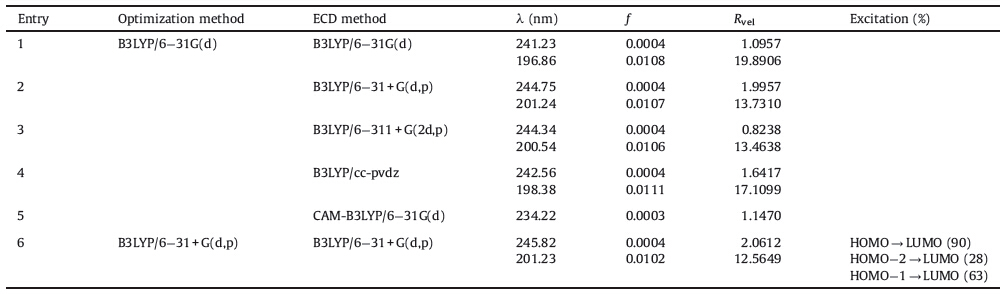
Artemisinin (1,Fig. 1) and its derivatives are well-known for their remarkable antimalaria activity,and they are the worldwide standard therapy against Plasmodium falciparum malaria [1]. Besides antimalaria activity,artemisinin has been shown to possess various pharmacological actions,such as anti-cancer, anti-fungal,and anti-inflammatory [2]. Artemisinin was first isolated from Artemisia annua,a plant used in Chinese traditional medicine. Apart from isolation from plants,artemisinin can also be produced in large scale using genetically engineered yeast [3]. Artemether (2,Fig. 1) is a methyl ether derivative of artemisinin and possesses higher antimalaria activity. Artemisinin is a sesquiterpene d-lactone with a special peroxide group,which is regarded to be responsible for kinds of biological activities. Because of the particularly of the peroxide group,chemical structures of artemisinin and its derivatives still attracted great interest from chemists and medicinal chemists.
|
Download:
|
| Fig. 1.Chemical structures of artemisinin (1),artemether (2) and 1- deoxyartemisinin (3). | |
Chiroptical properties including electronic circular dichroism (ECD) and optical rotational dispersion (ORD) utilize different effects of chiral compounds on the left- and right-handed polarized light to study stereochemical characteristics. They are thought as an efficient alternative way to X-ray crystallization in the field of stereochemistry,but the interpretation of Cotton effects often hindered its wide application in the past. As shown in Fig. 1,there are several chiral centers in chemical structures of 1 and 2. It should be emphasized that chiroptical properties played a great role on the primary absolute configuration establishment of 1. In the first report on the structure and reaction of 1,it was stated that its chemical formula and relative configuration was identified by X-ray diffraction. Meanwhile,its absolute configuration was firstly deduced by comparison of ORD curve of 1 in chloroform with a trans-lactone from arteannuin B and using empirical lactone rule [4]. Then,the assignment was further verified by anomalous dispersion of Cu-radiation by oxygen atoms and total synthesis [5, 6]. In 1982,Professor Liang reported ECD spectra of several artemisinin derivatives and proposed the maximum at 260 nm might be caused by overlay of ECD bands from δ-lactone and peroxide groups [7]. Nearly ten years later,they studied the correlation between the absolute configuration and Cotton effect of organic peroxide using CH3-O-O-CH3 as a model molecule [8].
Nowadays,with the rapid development of time-dependent density functional theory (TDDFT) and computer technology, chiroptical properties including ECD and ORD spectra of small molecules could be calculated theoretically with high accuracy and reliability [9, 10, 11]. This provides the feasibility to revisit chiroptical properties of 1 and 2 and the correlation with their stereochemical characteristics. However,up to our knowledge,no report was found to discuss theoretically ECD and ORD spectra of 1 and its derivatives as a full molecule.
Thus,as a part of our continuous effort to investigate chiroptical properties of natural products and chiral drugs,we studied chiroptical properties of compounds 1 and 2 using modern quantum-chemical calculation via TDDFT methodology. Herein, we will provide our results and discuss the ECD behavior of the peroxide bridge. 2. Experimental and computation
Standard samples of artemisinin (1) and artemether (2) were purchased from China National Institutes for Food and Drug Control. Their ECD spectra,at a concentration of 1.0-5.0 mg/mL in ethanol,were recorded in a quartz cuvette of 1 mm optical path length using a Jasco J-815 CD spectrometer (Jasco Inc.,Japan). Optical rotations of 1 and 2 were measured on a SGW-5 automatic polarimeter (INESA Inc.,Shanghai) in ethanol at different wavelengths (633,589,578,546,436 and 405 nm) and room temperature.
All quantum-chemical calculations were performed on structures with absolute configuration as depicted in Fig. 1. Initial conformational analysis was run using MMFF94 force field via the MOE softwarepackage [12]. The obtainedMMFF94conformerswere further optimized with the software Gaussian 09 using the B3LYP functional at the 6-31G(d) and 6-31 + G(d,p) basis set level [13]. These conformers were verified as minima on the potential energy surface by showing no imaginary frequency. Polarizable continuum model (PCM) was usedto consider solvent effects using the dielectric constants 24.85 for ethanol. Oscillator strengths and rotational strengths in dipole velocity (Rvel) representation of the 20 lowest electronic transitions were calculated for each conformer. ECD spectrawere then simulated by using aGaussian functionwith band width σ = 0.4 eV. ORD curves were obtained by calculating the specific optical rotations at different wavelengths using the length representation together with gauge-invariant atomic orbitals. 3. Results and discussion
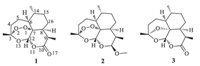
Because of the skeleton rigidity,both 1 and 2 have only one stable conformer in the equilibrium mixture. The optimized geometries at B3LYP/6-31 + G(d,p) level are shown in Fig. 2. When compared with the single crystal X-ray data,the computed bond parameters coincided very well to the available experimental data, with mean standard derivation of bond length being ±0.012Å[14]. The peroxide bridge is regarded necessary for the biological activity, and its bond strength correlates with the antimalaria activity [15]. The computed O-O bond lengths for 1 and 2 are 1.462 and 1.465Å, respectively,both located within the generally accepted range 1.458- 1.467Å. Two adjacent C-O bonds gave different bond lengths as 1.419 and 1.462Å,indicating the peroxide group was not strictly C2 symmetry. As to 1,the bond lengths of O1-O2-C3-O13-C12-O11- C10 chain are sequentially 1.462,1.419,1.449,1.394,1.459,and 1.356Å. The same carbon-oxygen chain in 2 gave a similar longshort- long bond length pattern. The nonplanar peroxide group of 1 arranged in a clockwise direction and gave a C-O-O-C dihedron angle as +47.2°,very close to the value +47.4° in single crystal. For 2,the C- O-O-C dihedron angle was also in a clockwise direction but the value decreased to +43.48. The good match of theoretical and experimental structural data demonstrated that the B3LYP hybrid functional and the 6-31 + G(d,p) basis set level could be a good choice to geometry analysis. The lower level of 6-31G(d) basis set could give similar results and need less computation time.
|
Download:
|
| Fig. 2.The optimized geometries of compounds 1-3 in ethanol. | |
Both compounds 1 and 2 have been included in the Chinese Pharmacopeia (2010 version),and specific optical rotation at Dline ([α]D) is listed as the testing item of identification [16]. The permissible ranges are +75°-+78° and +168°-+173° for 1 and 2 in ethanol,respectively. In this study,their experimental [α]D values were +76.7° and +169.8°,both located in the qualified range. Both compounds showed positive optical rotations and monotonically decreasing ORD curves with the increase of wavelength over the range 400-600 nm,but 1 gave smaller values than 2. Theoretical specific optical rotations of 1 and 2 were obtained using the B3LYP and CAM-B3LYP functionals,and the latter offered better results. The predicted [α]D values of 1 and 2 at CAM-B3LYP/6-31 + G(d,p) level are +104.43° and +204.45°,both in positive sign and very close to the experiment. Their theoretical ORD curves reproduced remarkably well the experimental data (Fig. 3). Thus,it is clear that correct absolute configurations of 1 and 2 could be assigned by comparison of the experimental and theoretical [α]D values and ORD curves.

|
Download:
|
| Fig. 3.Experimental and theoretical ORD curves of 1 and 2 at the CAM-B3LYP/6-31 + G(d,p) level in ethanol. | |
Experimentally,the ECD spectrum of 1 showed three ECD bands: positive ones at 260 and 200 nm,and a negative one at 230 nm (Fig. 4). Meanwhile,there is only one broad positive ECD band starting from 300 nmin the ECD spectrum of 2. ECD spectra of 1 and its derivatives attracted great interest because of the particularity of peroxide bond. It was Professor Liang who first reported ECD spectrum of 1 and ascribed the positive maximum at 260 nm to both lactone and peroxide groups. Then,Shen and co-worker [17] discussed ECD spectra of several artemisinin analogs and proposed that the contribution of peroxide chromophore was around 236 nm.

|
Download:
|
| Fig. 4.Experimental and theoretical ECD spectra of 1 and 2 at the B3LYP/6-31 + G(d,p) level in ethanol. | |
The theoretical oscillator and rotatory strengths of 1 and 2 were obtained at different levels using the optimized geometries and listed in Tables 1 and 2. It shown that the B3LYP functional could give similar results irrespective of the basis set level. However,the CAM-B3LYP functional would lose an important excitation state of the peroxide bridge at around 200 nm. For compound 2 with peroxide moiety as the only chromophore,two electron transition states with both positive rotatory strengths contributed to the broad positive ECD band in the far-UV range from 300 nm to 200 nm. There are two chromophores peroxide bridge and δ-lactone group in the chemical structure of 1. The positive rotatory strengths at 243 and 201 nm are due to the peroxide group,and the negative Cotton effect at 221 nm was originated from the n→π* transition of the carbonyl group of d-lactone. The predicted ECD spectrum of 1 showed the same tendency as the experiment,with positive-negative-positive mode from the long wavelength. Therefore,it is undoubted that correct absolute configurations of 1 and 2 could be achieved by comparison of the experimental and theoretical ECD profiles.
| Table 1 Computed absorption wavelengths (>195nm) and rotational strengths for 1 at different levels of basis sets. |
| Table 2 Computed absorption wavelengths (>195nm) and rotational strengths for 2 at different levels of basis sets. |
To clearly illustrate the effect of the peroxide bridge on ECD spectrum,1-deoxyartemisinin (3,Fig. 1) with only a lactone chromophore was selected to investigate. ECD calculation of 3 gave a negative rotatory strength at 220 nm,which corresponded to the negative Cotton effect at 220 nm observed experimentally [17]. As shown in Fig. 2,the β-carbon atoms of 1 and 3 were below the d-lactone plane,which would lead to a negative Cotton effect in absorption wavelength of the n→π* transition of the carbonyl group according to the Beecham rule [18]. Our results verified the suitability of this empirical rule in the case of 1 and 3,which stated the sign of the n→π* Cotton effect was solely determined by the position of the β-carbon atom relative to the lactone plane. It is interesting that the sum of the computed ECD spectra of 2 and 3 gave a better coincidence with the experimental data of 1 than the calculated data of 1,which reproduced all the key Cotton effects but a little blue-shifted (Fig. 5). It could attribute to the 5 nm blueshift of absorption wavelength of 1 compared with 2. This indicates that ECD spectra possess additivity,and it needs the development of new hybrid functional to calculate excitation energies of complex systems with higher accuracy.
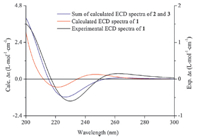
|
Download:
|
| Fig. 5.Sum of calculated ECD spectra of 2 and 3 and experimental ECD spectra of 1. | |
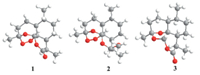
Molecular orbitals involved in key transitions for ECD spectra of 1 and 2 are shown in Fig. 6. For 2,the positive rotatory strength at 245 nm is originated from the MO81 (HOMO)→MO82 (LUMO) transition,which is an n→σ* transition of the peroxide bridge. Meanwhile,themoreintensepositive rotatory strength at201 nmwas contributed by two transitions from MO79 (HOMO-2) and MO80 (HOMO-1) to LUMO.Thesetwotransitionwere also n→σ* transition with lone pair electrons of all oxygen atoms participating in the molecule orbitals.As to 1,threelowest-energy rotatory strengthswere all originated from the n→σ* transition of the peroxide bridge and n→π* transition of the lactone group. It seems that interactionmight take place between the lone pair electrons of these two chromophores and lead to the delocalization of the unshared pair electrons of all oxygen atoms in the carbon-oxygen chain. This might interpret the high thermal stability of 1 and its analogs.

|
Download:
|
| Fig. 6.Molecular orbitals involved in the key electron transitions of 1 and 2 in ethanol (isosurface value of 0.05 au). | |
It was deduced empirically that the helix direction of the C-O- O-C torsion might relate to the sign of the peroxide Cotton effect. Huang reported ab initio calculation of the peroxide bond using MRDCI method and DZP basis set,but only the fragment CH3-O- O-CH3 instead of full molecule was studied [19]. Since the peroxide bridge might interact with other chromophores to give a complex ECD profile,a systematic investigation of this correlation in various natural peroxides using TDDFT methodology is underway. 4. Conclusion
In conclusion,ECD bands of 1 and 2 have been interpreted theoretically using modern quantum-chemical calculation via TDDFT methodology. The peroxide group can produce two significant rotatory strengths in the far-UV range due to the lowest-energy electronic transitions of HOMO→LUMO/LUMO + 1 for 1,and HOMO-2/HOMO-1/HOMO→LUMO for 2. Interaction might occur between the lone pair electrons of the peroxide bridge and lactone. This work also showed that the absolute configuration assignments of 1 and 2 could be unambiguously achieved by comparison of experimental and theoretical data.
AcknowledgmentsAll quantum computations were carried out using Gaussian 09 package,on an IBM cluster machine located at the High Performance Computing Center of Peking Union Medical College. This study was supported by the Fundamental Research Funds for the Central Institutes of China (No. 2012ZD03).
| [1] | N.M. Douglas, N.M. Anstey, B.J. Angus, F. Nosten, R.N. Price, Artemisinin combination therapy for vivax malaria, Lancet Infect. Dis. 10 (2010) 405-416. |
| [2] | W.E. Ho, H.Y. Peh, T.K. Chan, W.S. Wong, Artemisinins: pharmacological actions beyond antimalarial, Pharmocol. Therapeut. 142 (2014) 126-139. |
| [3] | D.K. Ro, E.M. Paradise, M. Ouellet, et al., Production of the antimalarial drug precursor artemisinic acid in engineered yeast, Nature 440 (2006) 940-943. |
| [4] | J.M. Liu, M.Y. Ni, J.F. Fan, et al., Structure and reaction of Arteannuin, Acta Chim. Sin. 37 (1979) 129-143. |
| [5] | Qinghaosu Research Group, Crystal structure and absolute configuration of Qinghaosu, Sci. Sin. 11 (1979) 1114-1128. |
| [6] | G. Schmid, W. Hofheinz, Total synthesis of Qinghaosu, J. Am. Chem. Soc. 105 (1983) 624-625. |
| [7] | X.T. Liang, Circular dichroism of the peroxidic linkage, Acta Chim. Sin. 40 (1982) 287-288. |
| [8] | J.J. Liu, G.L. Duan, X.T. Liang, An ab initio study on the correlation between the absolute configuration and the CD spectra of organic peroxides, Chin. Chem. Lett. 2 (1991) 245-248. |
| [9] | P.L. Polavarapu, Renaissance in chiroptical spectroscopic methods for molecular structure determination, Chem. Rec. 7 (2007) 125-136. |
| [10] | N. Berova, L.D. Bari, G. Pescitelli, Application of electronic circular dichroism in configurational and conformational analysis of organic compounds, Chem. Soc. Rev. 36 (2007) 914-931. |
| [11] | L. Li, C. Li, Y.K. Si, D.L. Yin, Absolute configuration of Buagafuran: an experimental and theoretical electronic circular dichroism study, Chin. Chem. Lett. 24 (2013) 500-502. |
| [12] | MOE 2009.10, Chemical Computing Group Inc., http://www.chemcomp.com. |
| [13] | Gaussian 09, revision C.01, Gaussian, Inc., http://www.gaussian.com. |
| [14] | J.N. Lisgarten, B.S. Potter, C. Bantuzeko, R.A. Palmer, Structure, absolute configuration, and conformation of the antimalarial compound, Artemisinin, J. Chem. Crystallogr. 28 (1998) 539-543. |
| [15] | I. Fernandez, A. Robert, Peroxide bond strength of antimalaria drugs containing an endoperoxide cycle. Relation with biological activity, Org. Biomol. Chem. 9 (2011) 4098-4107. |
| [16] | The State Pharmacopoeia Committee of China, The Pharmacopoeia of the People's Republic of China, China Medical and Technology Press, Beijing, 2010. |
| [17] | C.Y. Shen, Y. Li, Circular dichroism study of Qinghaosu and its derivatives, Acta Chim. Sin. 49 (1991) 183-186. |
| [18] | A.F. Beecham, Circular dichroism in lactones, Tetrahedron Lett. 9 (1968) 2355-2360. |
| [19] | M.B. Huang, H.U. Suter, Ab initio study of dimethyl peroxide, J. Mol. Struct. Theochem. 337 (1995) 173-178. |