The [1, 6]naphthyridine and their benzo-fused analogs are an important pharmacophore present in many natural [1] and designed synthetic products of therapeutic importance. They are associated with a wide spectrum of biological activities ranging from anticancer [2],anti-HIV-1 [3],antimicrobial [4] and cytotoxic activity [5]. Therefore,the synthesis of [1, 6]naphthyridine derivatives has aroused great interest in organic and medicinal communities [6, 7, 8, 9, 10].
Earlier reports for the synthesis of benzo[b][1, 6]naphthyridines has been carried out eithervia Vilsmeir-Haack reaction of 2-hydroxy-4-arylaminopyridines [11] orviaDiels-Alder process of Mannich base of 4-hydroxy-2-quinolone with anilines [12]. Deady et al.,have reported that synthesis of the benzo[b][1, 6]naphthyridine-4-carboxylic acids by multi-step reactions of (3-carboxyquinolin-2-yl)acetate with Vilsmeier reagent [13]. Recently,Singh and coworkers reported palladium-catalyzed synthesis of benzo[b][1, 6]naphthyridinesviaSonogashira coupling and annulation reactions from 2-chloroquinoline-3-carboxaldehydes or 2-chloroquinoline-3-carbonitriles with terminal alkynes [9]. As a result, the development of simple,straightforward and efficient methods for synthesis of benzo[b][1, 6]naphthyridine derivatives is strongly desirable.
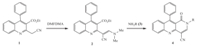
In a continuation of our interest in the synthetic methodologies of fused heterocycles [14],herein we report the facile synthesis of 1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyridine-4-carbonitriles (4) by a tandem addition-elimination-cyclization reaction of ethyl a-(dimethylaminomethylene)-2-cyanomethyl-4-phenylquinoline-3-carboxylate (2) with primary amines (3) (Scheme 1).
|
Download:
|
| Scheme 1. Syntheses of 1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyridine-4-carbonitriles. | |
To a solution of ethyl 2-cyanomethyl-4-phenylquinoline-3-carboxylate 1 [15] (6.3 g,20.0 mmol) in DMF (20 mL) was added DMFDMA (3.8 g,30.0 mmol) and the mixture was heated at 100°C for 2 h. After cooling to room temperature,then water (50 mL) was added and stirred for 20 min. The solid was filtered and recrystallized from EtOH to give 6.5 g (87%) of 2,as deep red prism. Mp 150-151°C. IR (KBr,cm-1 ):n2234 (CN),1672 (C==O). 1H NMR (400 MHz,CDCl3): δ 1.63 (t,3H,J= 7.2 Hz),3.40 (s,3H), 3.43 (s,3H),4.62 (q,2H,J= 7.2 Hz),7.20-7.25 (m,2H),7.43-7.50 (m,5H),7.89-7.90 (m,1H),7.85 (s,1H),8.32 (d,1H,J= 10.4 Hz). Anal. Calcd. for C23H21N3O2: C 74.37,H 5.70,N 11.31. Found: C 74.49,H 5.86,N 11.48. 2.2. Preparation of 2-substituted 1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyridine-4-carbonitrile derivatives
A mixture of ethyl a-(dimethylaminomethylene)-2-cyanomethyl-4-phenyl-quinoline-3-carboxylate (2,1.0 mmol),amine (3,1.0 mmol) in 25 mL HOAc-DMF (1:1,v:v) was heated to reflux under stirring for 6-11 h. At the end of the reaction,cooled the mixture to room temperature,added water (20 mL) to the mixture and stirred for 30 min. The solid was filtered and recrystallized to afford the corresponding products (4a-l).
4a:Mp>300°C. IR (KBr,cm-1 ): n2250 (CN),1680 (C==O). 1H NMR (400 MHz,CDCl3): δ 3.50 (s,3H),7.23-7.24 (m,2H), 7.47-7.49 (m,5H),7.84-7.88 (m,1H),7.97 (s,1H),8.29 (d,1H, J= 10.4 Hz). 13C NMR (100 MHz,CDCl3): δ 37.4,94.1,115.6,116.1, 126.5,127.1,127.2,127.5,127.8,128.6,128.8,129.3,132.5,143.7, 144.6,149.4,150.2,161.5. Anal. Calcd. for C20H13N3O: C 77.16, H 4.21,N 13.50. Found: C 77.25,H 4.37,N 13.68.
4b:Mp>300°C. IR (KBr,cm-1 ): n2228 (CN),1685 (C==O). 1H NMR (400 MHz,CDCl3): δ 1.32 (t,3H,J= 6.4 Hz),3.95 (q,2H, J= 6.4 Hz),7.19-7.21 (m,2H),7.46-7.54 (m,5H),7.86-7.88 (m, 1H),7.97 (s,1H),8.29 (d,1H,J= 8.8 Hz). 13C NMR (100 MHz, CDCl3): δ 14.4,44.9,94.1,115.8,116.3,126.4,127.1,127.2,127.5, 127.8,128.5,128.7,128.8,129.3,129.4,132.4,143.5,149.3,160.7. Anal. Calcd. for C21H15N3O: C 77.52,H 4.65,N 12.91. Found: C 77.68,H 4.79,N 13.04.
4c: Mp 260-262°C. IR (KBr,cm-1 ):n2234 (CN),1681 (C==O). 1H NMR (400 MHz,CDCl3): δ 0.93 (t,3H,J= 6.4 Hz),1.69-1.75 (m,2H),3.84 (q,2H,J= 6.4 Hz),7.21-7.22 (m,2H),7.44-7.54 (m,5H),7.84-7.87 (m,1H),7.95 (s,1H),8.28 (d,1H,J= 8.8 Hz). 13C NMR (100 MHz,CDCl3): δ 10.6,22.5,51.4,94.8,115.8,116.3, 126.3,127.2,127.3,127.4,128.6,128.8,129.1,143.2,144.2,145.1, 149.3,150.2,154.0,160.8. Anal. Calcd. for C22H17N3O: C 77.86, H 5.05,N 12.38. Found: C 77.97,H 5.17,N 12.49.
4d: Mp 295~297°C. IR (KBr,cm-1 ):n2251 (CN),1675 (C==O). 1H NMR (400 MHz,CDCl3): δ 0.92 (t,3H,J= 6.4 Hz),1.30-1.34 (m, 2H),1.66-1.67 (m,2H),3.87 (q,2H,J= 6.4 Hz),7.21-7.23 (m,2H), 7.43-7.54 (m,5H),7.83-7.86 (m,1H),7.96 (s,1H),8.28 (d,1H, J= 8.8 Hz). 13C NMR (100 MHz,CDCl3): δ 13.3,19.9,31.3,49.8,93.8, 115.8,116.3,126.3,127.1,127.4,128.6,128.8,129.1,137.4,143.3, 144.2,145.2,149.3,150.2,153.9,160.8. Anal. Calcd. for C23H19N3O: C 78.16,H 5.42,N 11.89. Found: C 78.32,H 5.58,N 11.97.
4e:Mp>300°C. IR (KBr,cm-1 ): n2241 (CN),1683 (C==O). 1H NMR (400 MHz,CDCl3): δ 1.17-1.24 (m,1H),1.33-1.52 (m,4H), 1.70-1.73 (m,1H),1.87-1.89 (m,4H),4.75-4.82 (m,1H),7.21-7.24 (m,2H),7.44-7.46 (m,2H),7.54-7.56 (m,3H),7.84-7.85 (m,1H), 7.80 (s,1H),8.28 (d,1H,J= 8.8 Hz). 13C NMR (100 MHz,CDCl3): δ 25.0,25.6,32.0,32.5,53.3,54.7,93.9,116.1,116.2,126.3,126.9, 127.1,127.4,127.5,128.9,132.4,137.7,139.9,140.9,141.7,148.4, 150.2,160.8. Anal. Calcd. for C25H21N3O: C 79.13,H 5.58,N 11.07. Found: C 79.28,H 5.74,N 11.16.
4f:Mp>300°C. IR (KBr,cm-1 ): n2231 (CN),1687 (C==O). 1H NMR (400 MHz,CF3CO2D): δ 5.22 (s,2H),7.24-7.28 (m,4H), 7.36-7.37 (m,3H),7.61-7.66 (m,3H),7.87-7.88 (m,2H),8.32-8.37 (m,1H),8.56 (d,1H,J= 8.4 Hz),8.70 (s,1H). 13C NMR (100 MHz, CF3CO2D): δ 83.2,111.4,115.9,118.9,119.2,125.8,126.6,128.2, 128.3,128.5,128.7,129.1,129.6,133.3,139.3,140.2,140.4,144.9, 152.1,152.4,158.9,168.7. Anal. Calcd. for C26H17N3O: C 80.60, H 4.42,N 10.85. Found: C 80.73,H 4.58,N 10.95.
4g: Mp 287-289°C. IR (KBr,cm-1 ):n2242 (CN),1676 (C==O). 1H NMR (400 MHz,CDCl3): δ 7.15-7.26 (m,4H),7.48-7.52 (m,8H), 7.87-7.90 (m,1H),8.06 (s,1H). 8.34 (d,1H,J= 9.2 Hz). 13C NMR (100 MHz,CDCl3): δ 94.2,113.2,115.0,116.3,124.5,125.6,126.3, 128.6,129.1,130.1,130.4,130.5,130.8,133.7,139.5,140.3,140.6, 145.3,152.4,159.6,161.5. Anal. Calcd. for C25H15N3O: C 80.41, H 4.05,N 11.25. Found: C 80.56,H 4.16,N 11.38.
4h: Mp 295-297°C. IR (KBr,cm-1 ):n2225 (CN),1680 (C==O). 1H NMR (400 MHz,CDCl3): δ 2.12 (s,3H),7.17-7.28 (m,6H), 7.46-7.51 (m,5H),7.89-7.91 (m,1H),7.93 (s,1H). 8.33 (d,1H, J= 9.2 Hz). 13C NMR (100 MHz,CDCl3): δ 17.9,94.5,115.5,116.6, 126.6,126.7,126.8,127.0,127.3,127.5,127.7,128.6,129.4,130.4, 130.6,132.6,137.0,143.5,144.4,145.6,149.4,150.3,160.4. Anal. Calcd. for C26H17N3O: C 80.60,H 4.42,N 10.85. Found: C 80.74, H 4.54,N 10.93.
4i:Mp>300°C. IR (KBr,cm-1 ): n2257 (CN),1673 (C==O). 1H NMR (400 MHz,CDCl3): δ 2.36 (s,3H),7.08-7.18 (m,2H),7.21- 7.23 (m,3H),7.47-7.48 (m,5H),7.86-7.87 (m,1H),8.03 (s,1H), 8.32 (d,1H,J= 8.8 Hz). 13C NMR (100 MHz,CDCl3): δ 21.5,94.4, 115.6,116.6,124.6,126.5,126.9,127.1,127.2,127.4,127.9,128.7, 129.3,130.2,131.9,132.6,133.8,143.7,144.8,145.6,149.3,150.2, 160.8. Anal. Calcd. for C26H17N3O: C 80.60,H 4.42,N 10.85. Found: C 80.72,H 4.57,N 10.96.
4j:Mp>300°C. IR (KBr,cm-1 ): n2242 (CN),1670 (C==O). 1H NMR (400 MHz,CF3CO2D): δ 2.39 (s,3H),7.18 (d,2H,J= 8.4 Hz), 7.26-7.27 (m,2H),7.33 (d,2H,J= 8.4 Hz),7.58-7.60 (m,3H),7.91- 7.96 (m,2H),8.38-8.39 (m,1H),8.63 (d,1H,J= 8.8 Hz),8.75 (s,1H). 13C NMR (100 MHz,CF3CO2D): δ 20.7,113.2,115.0,116.0,116.2, 124.9,125.7,126.5,128.5,129.0,130.1,130.3,130.6,130.9,133.2, 139.4,140.5,140.7,145.1,152.9,159.5,169.2. Anal. Calcd. for C26H17N3O: C 80.60,H 4.42,N 10.85. Found: C 80.75,H 4.51,N 10.98.
4k:Mp>300°C. IR (KBr,cm-1 ): n2234 (CN),1679 (C==O). 1H NMR (400 MHz,CDCl3): δ 2.35 (s,3H),7.16-7.22 (m,6H), 7.44-7.48 (m,5H),8.88-8.89 (m,1H),8.03 (s,1H),8.32 (d,1H, J= 8.8 Hz). 13C NMR (100 MHz,CDCl3): δ 56.5,94.4,114.0,115.6, 116.6,126.5,127.3,127.4,127.9,128.6,128.8,129.3,130.6,132.6, 133.8,143.9,145.0. 145.8,149.4,150.3,154.5,161.0. Anal. Calcd. for C26H17N3O2: C 77.41,H 4.25,N 10.42. Found: C 77.53,H 4.38, N 10.57.
4l:Mp>300°C. IR (KBr,cm-1 ): n2248 (CN),1674 (C==O). 1H NMR (400 MHz,CDCl3): δ 7.11-7.15 (m,2H),7.20-7.22 (m,2H), 7.28-7.29 (m,2H),7.48-7.49 (m,5H),7.88-7.90 (m,1H),8.01 (s, 1H),8.32 (d,1H,J= 8.8 Hz). 13C NMR (100 MHz,CDCl3): δ 95.0, 115.4,115.9,116.2,116.5,117.3,117.5,126.7,127.0,127.3,127.5, 128.0,128.7,128.8,129.6,132.8,143.2,144.2,149.1,150.3,160.8. Anal. Calcd. for C25H14FN3O: C 76.72,H 3.61,N 10.74. Found: C 76.86,H 3.79,N 10.86. 3. Results and discussion
The chemistry of enamines and their derivatives has numerous attractive features that have made them important building blocks in current organic trends. Over the decades,enamines have been used for the synthesis of a wide variety of heterocyclic compounds[16].
The authors demonstrated that the 1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyridine-4-carbonitriles can be readily synthesized from 2-cyanomethylquinoline-3-carboxylate by treatment with dimethylformamide dimethylacetal (DMFDMA),followed by cyclization of the intermediate enamine by attack of primary amine (Scheme 1).
In this study,the key intermediate enamine,synthesis for 1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyridines,ethyl α-(dimethylamino methylene)-2-cyanomethyl-4-phenylquinoline-3-carboxylate 2 was obtained by the condensation of ethyl 2-cyanomethyl-4-phenyl quinoline-3-carboxylate1with DMFDMA,in 87% yield. Its structure was determined from the spectral data as well as elemental analysis (C23H21N3O2). The 1H NMR spectrum shows singlet peak atd 3.40 (s,3H,NCH3),3.43 (s,3H,NCH3) and 7.85 (s, 1H) for dimethyl-aminoacrylonitrile.
In an initial endeavor,we selected methylamine 3a as model primary amine to react with equimolar amounts of intermediate enamine 2 for the preparation of 1-oxo-1,2-dihydrobenzo[b][1, 6]-naphthyridine4aand investigated the optimal reaction conditions.
The synthesis of 4a was assessed for the effect of solvents on yields. Several single organic solvents (e.g.,ethanol,glacial acetic acid,DMF) were tested. The results are summarized in Table 1.
| Table 1 Optimization of reaction conditions on the synthesis of 1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyridine 4a.a |
As shown in Table 1,the reaction which proceeded in a mixture of glacial acetic acid with DMF (1:1,v:v) (entry 5) resulted in higher yield and was thus chosen as the solvent for subsequent reactions. To further optimize reaction conditions,the same reaction was carried out in the mixed solvent of glacial acetic acid with DMF (1:1,v:v) at temperatures ranging from 110 to 140°C (entries 7-9). We found the yield of 4a improved and the reaction time shortened,as the temperature was increased from 110 to 120°C (entries 6 and 7). Further increase of the temperature above 120°C has led to decrease on the yield of 4a (entries 8 and 9). Therefore, 120°C was chosen as the optimal reaction temperature for all further reactions.
Under the optimized conditions,a wide range of primary amines 3 underwent this one-pot condensation with ethyla-(dimethylaminomethylene)-2-cyanomethyl-4-phenylquinoline-3-carboxylate2to give the corresponding 1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyridines 4 .
As shown in Table 2,the reaction was successful for primary amines 3 incorporating alkyl (entries 1-6),and aromatic (entries 7-12) R groups carrying either electron-donating or electron withdrawing substituents reacted efficiently giving good yields (80%-94%).
| Table 2 Synthesis of 1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyrdines 4. |
All the products were characterized by IR, 1H NMR, 13C NMR and elemental analysis. And all the data is consistent with the desired structures.
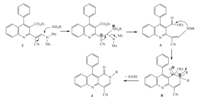
The proposed mechanism of the process is summarized in Scheme 2. The sequence involves an initial conjugate addition of the amine 3 on the double bond of the enamine 2 accompanied with the departure of dimethylamine,followed by an intramolecular cyclization reaction between the nitrogen of the formed secondary amine A and the carbonyl of the ester group to give B. Finally,the reaction ends up with the elimination of ethanol forming1-oxo-1,2-dihydrobenzo[b][1, 6]naphthyridine-4-carbonitriles 4.
|
Download:
|
| Scheme 2. A proposed mechanism for the formation of 4. | |
In conclusion,we have successfully developed and efficient method for the synthesis of 1-oxo-1,2-dihydrobenzo[b][1, 6] naphthyridine-4-carbonitrile derivatives via tandem addition- elimination-cyclization reaction of a-(dimethylaminomethylene)-2-cyanomethyl-4-phenylquinoline-3-carboxylate with primary amines. The ready accessibility of the starting materials and the generality of this process make the reaction highly valuable in view of the synthetic and medicinal importance of a multiheterocyclic framework of this type.
AcknowledgmentWe are grateful for financial support from the Science and Technology Department of Liaoning Province (No. 2011220022).
| [1] | H. Nakamura, J. Kobayashi, Y. Ohizumi, Isolation and structure of aaptamine a novel heteroaromatic substance possessing a-blocking activity from the sea sponge Aaptos, Tetrahedron Lett. 23 (1982) 5555-5558. |
| [2] | J.J. Bowling, H.K. Pennaka, K. Ivey, et al., Antiviral and anticancer optimization studies of the DNA-binding marine natural product aaptamine, Chem. Biol. Drug Des. 71 (2008) 205-215. |
| [3] | W. Gul, N.L. Hammond, M. Yousaf, et al., Modification at the C9 position of the marine natural product isoaaptamine and the impact on HIV-1, mycobacterial, and tumor cell activity, Bioorg. Med. Chem. 14 (2006) 8495-8505. |
| [4] | G.R. Pettit, H. Hoffmann, J. McNulty, et al., Antineoplastic agents 380. Isolation and X-ray crystal structure determination of isoaaptamine from the republic of Singapore Hymeniacidon sp. and conversion to the phosphate prodrug hystatin 1, J. Nat. Prod. 67 (2004) 506-509. |
| [5] | L.W. Deady, M.L. Rogers, L. Zhuang, et al., Synthesis and cytotoxic activity of carboxamide derivatives of benzo[b][1 6]naphthyridin-(5H)ones, Bioorg. Med. Chem. 13 (2005) 1341-1355. |
| [6] | (a) E.L. Larghi, M.L. Bohn, T.S. Kaufman, Aaptamine and related products. Their isolation, chemical syntheses, and biological activity, Tetrahedron 65 (2009) 4257-4282; (b) Y. Takahashi, T. Kubota, A. Shibazaki, et al., Nakijinamines C-E, new heteroaromatic alkaloids from the sponge Suberites species, Org. Lett. 13 (2011) 3016-3019; (c) C.X. Liu, X.L. Tang, P.L. Li, G.Q. Li, Suberitine A-D, four new cytotoxic dimeric aaptamine alkaloids from the marine sponge Aaptos suberitoides, Org. Lett. 14 (2012) 1994-1997. |
| [7] | C. Mukhopadhyay, P. Das, R.J. Butcher, An expeditious and efficient synthesis of highly functionalized [1,6]naphthyridines under catalyst-free conditions in aqueous medium, Org. Lett. 13 (2011) 4664-4667. |
| [8] | P.W. Phuan, M.C. Kozlowski, Convenient preparation of naphthyridines from halopyridines: sequential Heck coupling and cyclization, Tetrahedron Lett. 42 (2001) 3963-3965. |
| [9] | (a) A. Chandra, B. Singh, S. Upadhyay, et al., Copper-free Sonogashira coupling of 2-chloroquinolines with phenyl acetylene and quick annulation to benzo[b][1,6]-naphthyridine derivatives in aqueous ammonia, Tetrahedron 64 (2008) 11680-11685; (b) R.M. Singh, R. Kumar, N. Sharma, et al., Palladium-catalyzed one-pot synthesis of benzo[b][1,6]naphthyridines via Sonogashira coupling and annulation reactions from 2-chloroquinoline-3-carbonitriles, Tetrahedron 69 (2013) 9443-9450. |
| [10] | M. Piltan, I. Yavari, L. Moradi, Tandem synthesis of functionalized hexaalkyl benzoisoquinolinopyrrolonaphthyridine-hexacarboxylate, via isoquinoline based multi-component reaction, Chin. Chem. Lett. 24 (2013) 979-983. |
| [11] | C. Rivalle, E. Bisagni, Nouvelle synthe` se des pyrido[4,3-b]quinolé ines substitué es sur leur sommet 1,(New synthesis of pyrido[4,3-b]quinolines substituted at position 1), J. Heterocycl. Chem. 17 (1980) 245-248. |
| [12] | J.L. Asherson, D.W. Young, Synthesis of a variety of polycyclic heteroaromatic compounds via quinone methide intermediates, J. Chem. Soc. Chem. Commun. 24 (1977) 916-917. |
| [13] | (a) L.W. Deady, T. Rodemann, The reaction of homophthalic acid and some aza analogues with Vilsmeier reagent: a reinvestigation, J. Heterocycl. Chem. 38 (2001) 1185-1190; (b) L.W. Deady, T. Rodemann, L. Zhuang, Synthesis and cytotoxic activity of carboxamide derivatives of benzo[b][1,6]naphthyridines, J. Med. Chem. 46 (2003) 1049-1054; (c) L.W. Deady, M.L. Rogers, L. Zhuang, et al., Synthesis and cytotoxic activity of carboxamide derivatives of benzo[b][1,6]naphthyridin-(5H)ones, Bioorg. Med. Chem. 13 (2005) 1341-1355. |
| [14] | (a) D.L. Wang, S.F. Li, W. Li, et al., An efficient synthesis of 3-(guaiazulene-1-yl)succinimides by addition of guaiazulene to maleimides, Chin. Chem. Lett. 22 (2011) 789-792; (b) D.L. Wang, Q.T. Cui, S.S. Feng, et al., A new synthetic approach to azuleno[2,1-b]pyridine-4(1H)-ones, Heterocyles 85 (2012) 697-704; (c) D.L. Wang, Z. Dong, J. Xu, et al., An efficient synthesis of 2-(guaiazulen-1-yl)furan derivatives via intramolecular Wittig reactions, Chin. Chem. Lett. 24 (2013) 622-624; (d) D.L. Wang, Z. Dong, Q.T. Cui, et al., Synthesis of some pyrazole-fused pyrido[3,2-a]azulenes, Heterocyles 87 (2013) 2343-2350; (e) D.L. Wang, Z. Dong, Z. Liu, et al., Efficient one-pot synthesis of 1,4-dihydropyridino[3,2-c]coumarins, Chin. J. Org. Chem. 34 (2014) 783-787. |
| [15] | A.S. Degtyarenko, A.A. Tolmachev, D.A. Sysoiev, et al., Chlorotrimethylsilanemediated Friedländer synthesis of 2-(α-chloroalkyl)quinoline derivatives, Synthesis 24 (2007) 3891-3895. |
| [16] | (a) V. Kucklander, in: Z. Rappoport (Ed.), The Chemistry of Enamines, John Wiley & Sons, New York, 1994, pp. 525-639; (b) B. Stanovnik, J. Svete, Synthesis of heterocycles from alkyl 3-(dimethylamino) propenoates and related enaminones, Chem. Rev. 104 (2004) 2433-2480. |