Benzocyclobutenes are useful both as intermediates in the field of organic synthesis and as structural elements in active pharmaceutical compounds [1, 2]. More importantly,they can undergo thermal electrocyclic ring-opening to give ortho-xylylenes that can then react in pericyclic reactions,such as Diels-Alder cycloadditions [3, 4, 5]. These characteristics have been fully exploited in the total synthesis of polycyclic natural products [1, 2]. Although benzocyclobutenes possess high synthetic value themselves,hardly any general procedures exist for the preparation of functionalized benzocyclobutenes. This greatly restricts their availability and their use as organic synthesis intermediates.
The biaryl structural motif and 9,10-dihydrophenanthrene derivatives are dominant features and general structural units in many pharmaceutically important and biologically active compounds [6, 7]. As a consequence,organic chemists have attempted to exploit new and more efficient aryl-aryl bond-forming methods for over a century [8]. Despite the variety of routes that have been developed to synthesize aryl-aryl bonds,the most widespread methods are transition-metal-mediated reactions [9, 10]. Hence, synthesizing biaryl compounds and 9,10-dihydrophenanthrene derivatives in the absence of a transition metal catalyst remains an important and significant project.
Benzyne is an extremely reactive intermediate [11],and has been comprehensively used as a building block in organic synthesis [12]. Nevertheless,its application has been limited due to the strict reaction conditions for its preparation. With the emergence of moderate preparation methods of arynes [13],it has attracted a great deal of attention in the area of organic synthesis.
In this article,we report cycloaddition reactions of benzyne with olefins. These cycloaddition reactions provide effective routes for the synthesis of benzocyclobutenes,biaryl compounds and 9,10-dihydrophenanthrene derivatives in the absence of a transition metal catalyst. Moreover,these reactions display good yields. 2. Experimental
The benzyne precursor 1awas prepared using the known procedures [13]. Commercially available CsF,norbornadiene, norbornene,vinyl butyl ether,methyl acrylate,ethyl acrylate, styrene,trans-stilbene,indene and all reagents were used without further purification. The 1H NMR and 13C NMR spectra were acquired on American Varian Mercury Plus 400 spectrometer (400 MHz). The 1H NMR spectra are reported as follows: chemical shift in ppm (δ) relative to the chemical shift of TMS at 0.00 ppm, integration,multiplicities (s = singlet,d = doublet,t = triplet, m = multiplet),and coupling constant (Hz).13C NMR chemical shifts are reported in ppm (d) relative to the central line of the triplet for CDCl3at 77 ppm. EI-MS was obtained using a Thermo Scientific DSQII. Elemental analyses (C,H) were performed by the Microanalytical Services,College of Chemistry,CCNU. The X-ray crystal-structure determinations of 1c,8cand 9c were obtained on a Bruker APEX DUO CCD system. Column chromatographic separations were carried out on silica gel (200-300 mesh). TLC was performed using commercially prepared 100-400 mesh silica gel plates (GF254) and visualization was effected at 254 nm. 2.1. General synthetic procedure for 1c
The mixture of o-(trimethylsilyl)phenyl triflate (3 mmol, 2.0 equiv.),norbornadiene (1.0 equiv.),and anhydrous CsF (6.0 equiv.) was soluted in dried CH3CN (15 mL). Then,the resulting mixture was stirred at room temperature for 12 h. After the reaction completed,20 mL of water was added to the mixture, and then extracted with CH2Cl2 (3×20 mL),and dried over anhydrous Na2SO4and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: petroleum ether) to afford the desired product1c.
Compound 1c: White solid,mp: 121.8-122.58C. 1H NMR (400 MHZ,CDCl3):δ 7.19 (t,4H,J= 4 Hz),7.01-6.99 (m,4H),3.29 (s, 4H),2.38 (s,2H),0.76 (s,2H). 13C NMR (100 MHZ,CDCl3): δ 146.36, 127.32,121.96,49.41,37.28,26.04. EI-MS:m/z 244 [M]+ . Anal. Calcd. for C19H16: C,93.40; H,6.60. Found: C,93.48; H,6.55. 2.2. General synthetic procedure for 2c
The mixture of o-(trimethylsilyl)phenyl triflate (1 mmol, 1.0 equiv.),norbornadiene (2.0 equiv.),and anhydrous CsF (3.0 equiv.) was soluted in dried CH3CN (15 mL). Then,the resulting mixture was stirred at room temperature for 12 h. After the reaction completed,20 mL of water was added to the mixture, and then extracted with CH2Cl2 (3×20 mL),and dried over anhydrous Na2SO4and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: petroleum ether) to afford the desired product 2c.
Compound 2c: Colorless oil. 1H NMR (400 MHZ,CDCl3): δ 7.20- 7.15 (m,2H),7.09-7.05 (m,2H),6.24 (d,2H,J= 12 Hz),3.15 (t,2H, J= 10 Hz),2.78 (s,2H),1.27 (t,1H,J= 8 Hz),0.87 (d,1H,J= 8 Hz). 13C NMR (100 MHZ,CDCl3): δ 146.13,136.66,127.00,121.77,47.55, 41.54,41.34,29.70. EI-MS:m/z168 [M]+ . Anal. Calcd. for C13H12:C, 92.81; H,7.19. Found: C,92.85; H,7.12. 2.3. General synthetic procedures for 3c-6c
The mixture of o-(trimethylsilyl)phenyl triflate (3 mmol, 2.0 equiv.),olefins (1.0 equiv.),and anhydrous CsF (6.0 equiv.) was soluted in dried CH3CN (15 mL). The resulting mixture was stirred at room temperature for 12 h. After the reaction completed, 20 mL of water was added to the mixture,and then extracted with CH2Cl2 (3×20 mL),and dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: petroleum ether) to afford the desired products 3c-6c.
Compound 3c: Colorless oil. 1H NMR (400 MHZ,CDCl3):d7.20- 7.18 (m,2H),7.00-6.98 (m,2H),3.18 (s,2H),2.27 (s,2H),1.60-1.55 (m,2H),1.18 (d,2H,J= 4 Hz),0.97-0.94 (m,1H),0.85 (d,1H, J= 8 Hz). 13C NMR (100 MHZ,CDCl3): δ 146.50,127.11,121.81, 50.44,36.57,31.92,27.79. EI-MS:m/z= 170 [M]+ . Anal. Calcd. for C13H14: C,91.71; H,8.29. Found: C,91.65; H,8.33.
Compound 4c: Colorless oil. 1H NMR (400 MHZ,CDCl3): δ 7.28- 7.23 (m,2H),7.14 (d,2H,J= 8 Hz),5.04 (s,1H),3.66-3.57 (m,2H), 3.48-3.44 (m,1H),3.12 (d,1H,J= 12 Hz),1.65-1.57 (m,2H),1.44- 1.39 (m,2H),0.94 (t,3H,J= 6 Hz). 13C NMR (100 MHZ,CDCl3): δ 146.30,142.54,129.18,126.91,123.44,122.70,68.63,38.63,31.95, 19.37,13.90. EI-MS:m/z 176 [M]+ . Anal. Calcd. for C12H16O: C, 81.77; H,9.15. Found: C,81.81; H,9.09.
Compound 5c: Light yellow oil. 1H NMR (400 MHZ,CDCl3): δ 7.26-7.24 (m,2H),7.18 (d,1H,J= 4 Hz),7.11 (d,1H,J= 4 Hz),4.32 (t,1H,J= 4 Hz),3.73 (d,3H,J= 8 Hz),3.48 (d,2H,J= 4 Hz). These data are in good agreement with literature values [14].
Compound 6c: Light yellow oil. 1H NMR (400 MHZ,CDCl3): δ 7.26-7.23 (m,2H),7.18 (d,1H,J= 4 Hz),7.10 (t,1H,J= 8 Hz),4.30 (t,1H,J= 4 Hz),4.22-4.17 (m,2H),3.47 (t,2H,J= 4 Hz),1.32-1.27 (m,3H). 13C NMR (100 MHZ,CDCl3): d171.94,144.01,142.71, 127.91,127.09,122.67,122.23,60.58,45.74,33.68,14.04. EI-MS: m/z176 [M]+ . Anal. Calcd. for C11H12O2: C,74.98; H,6.86. Found: C, 74.91; H,6.91. 2.4. General synthetic procedure for 7c
The mixture of o-(trimethylsilyl)phenyl triflate (1.5 mmol, 3.0 equiv.),styrene (1.0 equiv.),and anhydrous CsF (9.0 equiv.) was soluted in dried 1,4-dioxane (5 mL). Then,the resulting mixture was stirred at 1108C for 12 h. After the reaction completed,20 mL of water was added to the mixture and then extracted with CH2Cl2 (3×20 mL),and dried over anhydrous Na2SO4and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: petroleum ether/CH2Cl2) to afford the desired product 7c.
Compound 7c: Light yellow oil. 1H NMR (400 MHZ,CDCl3): δ 7.75 (d,2H,J= 8 Hz),7.32-7.27 (m,3H),7.24 (d,3H,J= 4 Hz),2.89 (d,4H,J= 12 Hz).
13C NMR (100 MHZ,CDCl3): δ 137.13,134.30, 127.99,127.22,126.82,123.56,28.88. EI-MS:m/z180 [M]+ . Anal. Calcd. for C14H12: C,93.29; H,6.71. Found: C,93.25; H,6.76. 2.5. General synthetic procedure for 8c
The mixture of o-(trimethylsilyl)phenyl triflate (3 mmol, 3.0 equiv.),trans-stilbene (1.0 equiv.),and anhydrous CsF (9.0 equiv.) was soluted in dried CH3CN (15 mL). Then,the resulting mixture was stirred at room temperature for 12 h. After the reaction completed,20 mL of water was added to the mixture, and then extracted with CH2Cl2 (3×20 mL),and dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: petroleum ether/CH2Cl2) to afford the desired product 8c.
Compound 8c: White solid,mp: 165.8-166.6 °C. 1H NMR (400 MHZ,CDCl3):δ 7.91 (d,2H,J= 8 Hz),7.36-6.96 (m,16 H,arylH),4.43 (s,2H). 13C NMR (100 MHZ,CDCl3): δ 143.77,137.06, 134.25,129.95,128.29,128.13,128.03,127.54,126.26,123.52, 52.59. EI-MS:m/z332 [M]+ . Anal. Calcd. for C26H20: C,93.94; H, 6.06. Found: C,93.99; H,6.02. 2.6. General synthetic procedure for 9c
The mixture of o-(trimethylsilyl)phenyl triflate (3 mmol, 3.0 equiv.),indene (1.0 equiv.),and anhydrous CsF (9.0 equiv.) was soluted in dried CH3CN (15 mL). Then,the resulting mixture was stirred at room temperature for 12 h. After the reaction completed,20 mL of water was added to the mixture,and then extracted with CH2Cl2 (3×20 mL) and dried over anhydrous Na2SO4and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent: petroleum ether/CH2Cl2) to afford the desired product 9c.
Compound 9c: White solid,mp: 89.7-90.6 °C. 1H NMR (400 MHZ,CDCl3):δ7.27-7.18 (m,4H),7.13-7.06 (m,4H),6.41- 6.37 (m,1H),6.11 (d,1H,J= 8 Hz),5.98-5.95 (m,1H),5.88 (d,1H, J= 8 Hz),3.34 (d,2H,J= 20 Hz),1.83 (d,1H,J= 8 Hz),1.64-1.61 (m, 1H). 13C NMR (100 MHZ,CDCl3): δ 148.56,145.94,143.91,142.76, 131.37,128.24,127.80,127.69,127.57,125.88,125.00,124.32, 122.01,121.59,121.43,120.95,60.21,52.79,52.51,45.68, 45.44. EI-MS:m/z= 268 [M]+ . Anal. Calcd. for C21H16: C,93.99; H,6.01. Found: C,93.91; H,6.06. 3. Results and discussion
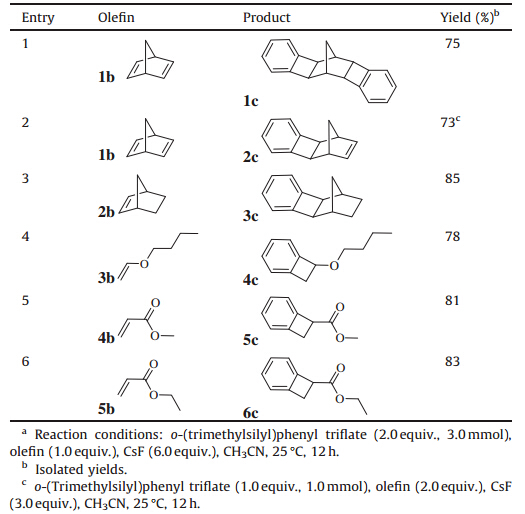
We initially examined the [2 + 2] cycloaddition reaction of benzyne and norbornadiene using 2.0 equiv. of compound 1a, 2.0 equiv. of 1b and 2.0 equiv. of CsF at 25 °C in anhydrous acetonitrile (see Table 1,entry 1),the desired [2 + 2] cycloaddition product1cwas obtained in a yield of 17%. In addition,we were able to grow a single crystal of 1c,and its structure was confirmed by Xray crystallography (Fig. 1a). Subsequently,we altered the molar ratio of the reactants and,as shown in Table 1,a higher yield was observed when the proportion of1bwas reduced from 2.0 to 1.0 equiv. (Table 1,entry 2). Increasing yields were also obtained when we increased the amount of CsF (Table 1,entries 3-4). Further studies concentrated on the effect of temperature. Data in Table 1 (entries 5 and 6) show that the reaction is less effective at higher temperatures. Therefore,the best result (75% yield of [2 + 2] cycloaddition reaction product 1c) was obtained using 1a (2.0 equiv.),1.0 equiv. of 1b,6.0 equiv. of CsF in acetonitrile at 25 °C for 12 h.
|
Download:
|
| Fig. 1. The single crystal structure of compound 1c(a),8c(b) and 9c(c) (hydrogen atoms are omitted for clarity). | |
| Table 1 Optimization of [2 + 2] cycloaddition reaction of benzyne with norbornadienea |
Using this optimized set of reaction conditions,we then tested other [2 + 2] cycloaddition reactions of benzyne and olefins (Table 2). It was determined that other olefin substrates,including norbornene 2b,vinyl butyl ether 3b,methyl acrylate 4b,ethyl acrylate 5b,could also smoothly undergo a [2 + 2] cycloaddition reaction with yields ranging from 78% to 85% (Table 2,entries 3-6). In addition,we obtained the product2cinvolving a single double bond [2 + 2] cycloaddition reaction of benzyne and norbornadiene in a yield of 73% using 1.0 equiv.1a,2.0 equiv.1band 3.0 equiv. CsF at 25 °C in anhydrous acetonitrile (Table 2,entry 2).
| Table 2 [2 + 2] Cycloaddition reaction products of benzyne with olefins.a |
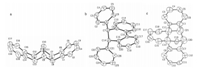
Next,biaryl compound 7c was obtained when we carried out the reaction of benzyne with styrene using 3.0 equiv. of 1a, 3.0 equiv. of 6b and 9.0 equiv. of CsF at 25 °C in anhydrous acetonitrile (Table 3,entry 1). Subsequently,we attempted to optimize the molar ratios of the reactants. As can be seen in Table 3, increasing yields were observed as the quantity of1bwas reduced from 3.0 equiv. to 1.5 equiv.,then to 1.0 equiv. under the same conditions (Table 3,entries 2-3). Subsequent studies focused on the effects of solvent and temperature. Entries 4-7 of Table 3 demonstrate that the best reaction condition used dioxane as solvent. Simultaneously,we also noticed that higher temperatures produced increasing yields (Table 3,entries 8-9). According to the above optimizations,the best result (87% yield of the biaryl compound 7c) was obtained using1a(3.0 equiv.),1.0 equiv. of 6b, 9.0 equiv. of CsF at 1108C in anhydrous dioxane.
| Table 3 Optimization of [2 + 4] cycloaddition reaction of benzyne with styrenea |
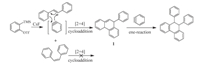
In later work,we obtained the remarkable cycloaddition product 8c in 62% yield when we examined the reaction of benzyne and trans-stilbene using 3.0 equiv. of1a,1.0 equiv. of 7b and 9.0 equiv. of CsF at 25 °C in anhydrous acetonitrile (Scheme 1). The possible mechanism for this reaction is shown in Scheme 2. The [2 + 4] cycloaddition reaction of trans-stilbene with benzyne generated fromo-(trimethylsilyl)phenyl triflate resulted in the generation of intermediate1,which can add to another molecule of electrophilic benzyne in a concerted-ene reaction leading to the formation of the target product. However,the [2 + 4] cycloaddition reaction ofcis-stilbene with benzyne can not be carried out due to the presence of the space steric hindrance. Therefore,we were not able to obtain the corresponding cycloaddition product under the same reaction conditions using cis-stilbene as substrate. The structure of compound 8c was determined by X-ray crystallography (Fig. 1b).
|
Download:
|
| Scheme 1. Cycloaddition reactions of benzyne with trans-stilbene. | |
|
Download:
|
| Scheme 2. Proposed mechanism of the reaction. | |
Finally,the reaction of benzyne and indene was investigated, and product 9c was obtained in a yield of 95% with 3.0 equiv. of 1a, 1.0 equiv. of 8b and 9.0 equiv. of CsF at 25 °C in anhydrous acetonitrile (Scheme 3). The synthesis of product 9c is the result of a [2 + 4] cycloaddition and then [2 + 2] cycloaddition reaction of benzyne. The structure of compound 9c has also been determined by X-ray crystallography (Fig. 1c).
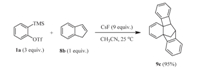
|
Download:
|
| Scheme 3. Cycloaddition reactions of benzyne with indene. | |
In summary,some novel and significant cycloaddition reactions and products based on the reactions of benzyne and olefins have been developed. These cycloaddition reactions display good yields, even in the absence of a transition metal catalyst. These reactions of benzyne with olefins provide efficient methods for the synthesis of benzocyclobutenes,biaryl compounds and 9,10-dihydrophenanthrene derivatives.
AcknowledgmentsThe authors acknowledge financial support from the National Natural Science Foundation of China (Nos. 20931006,21072070, 21072071,21272088),and the Program for Academic Leader in Wuhan Municipality (No. 201271130441).
| [1] | G. Mehta, S. Kotha, Recent chemistry of benzocyclobutenes, Tetrahedron 57 (2001) 625-659. |
| [2] | A.K. Sadana, R.K. Saini, W.E. Billups, Cyclobutarenes and related compounds, Chem. Rev. 103 (2003) 1539-1602. |
| [3] | W. Oppolzer, Intramolecular cycloaddition reactions of ortho-quinodimethanes in organic synthesis, Synthesis 11 (1978) 793-802. |
| [4] | J.L. Charlton, M.M. Alauddin, Orthoquinodimethanes, Tetrahedron 43 (1987) 2873-2889. |
| [5] | H. Nemoto, K. Fukumoto, Second generation of steroid synthesis via o-quinodimethane, Tetrahedron 54 (1998) 5425-5464. |
| [6] | F.D. Monache, G.D. Monache, J.F. Cavalcanti, et al., An unexpected dihydrophenanthrene from clusia paralycola, Tetrahedron Lett. 28 (1987) 563-566. |
| [7] | (a) K. Baba, T. Kido, M. Taniguchi, M. Kozawaqa, Stillbenoids from cassia garrettiana, Phytochemistry 36 (1994) 1509-1513; (b) M.H. Yang, L. Cai, M.H. Li, et al., Three new phenanthrenes from Monomeria barbata Lindl., Chin. Chem. Lett. 21 (2010) 325-328. |
| [8] | F. Ullmann, J. Bielecki, Ueber synthesen in der biphenylreihe, Chem. Ber. 34 (1901) 2174-2185. |
| [9] | S.P. Stanforth, Catalytic cross-coupling reactions in biaryl synthesis, Tetrahedron 54 (1998) 263-303. |
| [10] | J. Hassan, M. Sé vignon, C. Gozzi, E. Schulz, M. Lemaire, Aryl-aryl bond formation one century after the discovery of the Ullmann reaction, Chem. Rev. 102 (2002) 1359-1470. |
| [11] | (a) A. Bhunia, S.R. Yetra, A.T. Biju, Recent advances in transition-metal-free carbon-carbon and carbon-heteroatom bond-forming reactions using arynes, Chem. Soc. Rev. 41 (2012) 3140-3152; (b) J. Park, M. Yan, Covalent functionalization of graphene with reactive intermediates, Acc. Chem. Res. 46 (2013) 181-189; (c) A.T. Biju, N. Kuhl, F. Glorius, Extending NHC-catalysis: coupling aldehydes with unconventional reaction partners, Acc. Chem. Res. 44 (2011) 1182-1195; (d) A.M. Dyke, A.J. Hester, G.C. Lloyd-Jones, Organometallic generation and capture of ortho-arynes (Stuttgart), Synthesis 24 (2006) 4093-4112. |
| [12] | (a) H. Yoshida, K. Takaki, Multicomponent coupling reaction of arynes for construction of heterocyclic skeletons, Heterocycles 85 (2012) 1333-1349;(b) P.M. Tadross, B.M. Stoltz, A comprehensive history of arynes in natural product total synthesis, Chem. Rev. 112 (2012) 3550-3577; (c) C.M. Gampe, E.M. Carreira, Arynes and cyclohexyne in natural product synthesis, Angew. Chem. Int. Ed. 51 (2012) 3766-3778; (d) L. He, J.X. Pian, J. Zhang, Y.Z. Li, Highly efficient synthesis of 9-aminoxanthenes via the tandem reaction of arynes with salicyl N-tosylimines, Chin. Chem. Lett. 23 (2012) 1359-1362. |
| [13] | Y. Himeshima, T. Sonoda, H. Kobayashi, Fluoride-induced 1,2-elimination of otrimethylsilylphenyl triflate to benzyne under mild conditions, Chem. Lett. 12 (1983) 1211-1214. |
| [14] | M. Chaumontet, R. Piccardi, N. Audic, et al., Synthesis of benzocyclobutenes by palladium-catalyzed C-H activation of methyl groups: method and mechanistic study, J. Am. Chem. Soc. 130 (2008) 15157-15166. |