Several species of the genus Euphorbia (Euphorbiaceae) have long been used for the treatment of various diseases such as ascites,cancer,edema,and warts in China [1]. Phytochemical and biological investigations have revealed that plants of this genus are sources of various secondary metabolites with interesting chemical structures and significant bioactivities [2]. In particular, diterpenoids exert a variety of biological effects,such as antiinflammatory, anti-microbial,anti-proliferation,cytotoxic,and modulation of multidrug resistance activities [2]. Notably,ingenol mebutate from the sap of E. peplus has recently been approved in the United States,countries of the European Union,Australia,and Brazil for the treatment of actinic keratosis,a precursor to a form of squamous-cell carcinoma [3]. A nontumor-promoting 12-deoxytigliane diterpenoid from E. fischeriana and other species of Euphorbiaceae,prostratin,exhibits potent in vitro activity in inducing HIV expression in latently infected cell lines and primary cells. It has been advanced into preclinical trials,although the mechanism of action has not yet been completely elucidated [4]. This has promoted increased interest in research of diterpenoids in species of this genus [5, 6, 7, 8, 9].
As part of a program to access chemical diversity of Chinese traditional medicines and study their biological effects,especially focusing on minor components [10],we have investigated roots of Euphorbia micractina Boiss.,which is used in Chinese folk medicine for the treatment of tumors and warts [1]. In the previous study, more than 80 compounds with diverse structural features were characterized in fractions obtained from a root EtOH extract of this plant. Some of these compounds showed antiviral activity against HIV-1 replication,cytotoxic activity against the A2780 ovarian cancer cell line,or vascular-relaxing activity against phenylephrine- induced vasoconstriction [11, 12, 13, 14]. The investigation has been conducted of the remaining fraction with antiviral activity,led to the isolation of a minor diterpenoid (1),which possesses an unprecedented carbon skeleton (Fig. 1). Herein,we report details of the isolation,structure elucidation,postulated biogenetic pathway,and biological activity of this compound.
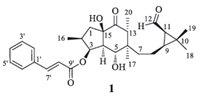
|
Download:
|
| Fig. 1.Structure of secoeuphoractin (1). | |
General experimental procedures: Optical rotation was measured on a PE Model 343. UV spectrum was measured on a JASCO V-650 spectrometer. CD spectrum was recorded on a JASCO J-815 CD spectrometer. IR spectrum was recorded on a Nicolet 5700 FTIR Microscope spectrometer (FT-IR Microscope Transmission). 1Dand 2D-NMR spectra were obtained at 600 MHz for 1H and 150 MHz for 13C,respectively,on an INOVA SYS 600 MHz spectrometer in acetone-d6 with solvent peaks as references. ESIMS data were measured with a Q-Trap LC/MS/MS (Turbo Ionspray source) spectrometer. HR-ESIMS data were,in turn, measured on an AccuToFCS JMS-T100CS spectrometer. Column chromatography was performed with silica gel (200-300 mesh, Qingdao Marine Chemical Inc.,Qingdao,China) and Sephadex LH- 20 (Pharmacia Biotech AB,Uppsala,Sweden). HPLC separation was performed on an instrument with a Waters 600 controller,a Waters 600 pump,and a Waters 2487 dual λ absorbance detector on a Prevail (250 mm × 10 mm i.d.) semi-preparative column packed with C18 (5 μm). Glass precoated silica gel GF254 plates were used for TLC. Spots were visualized under UV light or by spraying with 7% H2SO4 in 95% EtOH followed by heating.
Plant material: See refs. [11, 12, 13, 14].
Extraction and isolation: For extraction and preliminary fractionation of the extract,see refs. [11, 12, 13, 14]. Fraction A13 (5.5 g) was chromatographed over silica gel,eluting with a gradient of petroleum ether-acetone (10:1-1:1) to give subfractions (A13-1-A13-8). A13-5 (1.2 g) was subjected to flash column chromatography over reversed-phase C18 silica gel,eluting with a gradient of EtOH (0-95%) in H2O,to afford A13-5-1-A13-5-8. Fraction A13-5-5 (400 mg),which showed activity against HIV-1 replication with an inhibition rate of 38% at 10 mg/mL,was chromatographed over Sephadex LH-20 eluting with petroleum ether-CHCl3-MeOH (5:5:1),then purified with RP-HPLC using MeOH-H2O (3:1) as the mobile phase,to yield 1 (0.8 mg).
Secoeuphoractin (1): White amorphous powder,[α]D20 + 5.2 (c 0.08,MeOH); UV (MeOH) λmax (log ε) 216 (1.96),278 (1.99) nm; CD (MeOH): 215 (△ε -1.73),301 (△ε +0.77); IR (KBr,cm-1) vmax 3471, 3059,2962,2928,2875,2735,1716,1687,1634,1578,1540,1495, 1452,1380,1312,1281,1202,1185,1138,1071,1055,984,949, 866,807,768; 1H NMR (acetone-d6,600 MHz) and 13C NMR (acetone-d6,150 MHz),see Table 1; (+)-ESIMSm/z 483 [M+H]+,505 [M+Na]+,521 [M+K]+; HR-ESIMS m/z 483.2754 [M+H]+ (calcd. for C29H39O6,483.2741),505.2589 [M+Na]+ (calcd. for C29H38O29Na,505.2561).
| Table 1 NMR spectroscopic data for secoeuphoractin (1).a |
Compound 1 was obtained as a white amorphous solid. Its IR spectrum showed absorption bands assignable to hydroxy (3471 cm-1),carbonyl (1716 and 1687 cm-1),and aromatic ring (1578 and 1495 cm-1) functionalities. The positive ESIMS of 1 exhibited pseudo molecular ion peaks at m/z 483 [M+H]+,505 [M+Na]+,and 521 [M+K]+. HR-ESIMS at m/z 483.2754 [M+H]+ (calcd. for C29H39O6,483.2741) and 505.2589 [M+Na]+ (calcd. for C29H38O6Na,505.2561),together with the NMR data (Table 1), indicated the molecular formula as C29H38O6. The 1H NMR spectrum of 1 in acetone-d6 showed resonances attributed to an aldehyde group [δH 9.55 (d,1H,J = 5.4 Hz,H-12)],a cinnamoyl group [δH 7.70 (dd,2H,J = 7.8 and 1.8 Hz,H-2'/6'),7.45 (m,2H,H- 3'/5'),7.44 (m,1H,H-4'),7.78 (d,1H,J = 16.2 Hz,H-7'),and 6.75 (d, 1H,J = 16.2 Hz,H-8')],two oxymethines [δH 5.53 (m,1H,H-3) and 4.39 (dd,1H,J = 11.4 and 4.8 Hz,H-5)],three tertiary methyls [dH 1.31 (3H,s,H3-19),1.14 (s,3H,H3-18),and 0.65 (s,3H,H3-17)],two secondary methyl groups [δH 1.11 (d,3H,J = 7.2 Hz,H3-16) and 0.84 (d,3H,J = 7.2 Hz H3-20)]. It also showed signals assignable to two exchangeable hydroxy protons [δH 4.14 (s,OH-15) and 4.05 (d, J = 4.8 Hz,OH-5)] and partially overlapped resonances with complex coupling patterns due to aliphatic methylenes and methines between δH 1.0 and 3.5. Besides carbon signals attributable to the cinnamoyl group,the 13C NMR and DEPT spectra of 1 displayed 20 carbon resonances corresponding to five methyls,three methylenes,eight methines (two oxygen-bearing and an aldehyde),and four quaternary carbons (one oxygenbearing and a carbonyl) (Table 1). These spectroscopic data suggests that compound 1 is a cinnamic acid ester of an unusual tricyclic diterpenoid triol containing aldehyde and carbonyl groups [11, 12, 13, 14]. This was confirmed by 2D NMR data analysis.
The proton and proton-bearing carbon resonances in the NMR spectra of 1 were assigned by 1H-1H gCOSY and gHSQC spectroscopic data interpretation. In the 1H-1H gCOSY spectrum of 1,a series of homonuclear coupling correlations of H2-1 ↔ H- 2 ↔ H-3 ↔ H-4 ↔ H-5 ↔ OH-5 and H2-7 ↔ H2-8 ↔ H-9 ↔ H- 11 ↔ H-12 revealed the presence of two fragments with vicinal coupling chain extensions in 1 (Fig. 2,thick lines). The 1H-1H gCOSY correlation between H-2 and H3-16 and gHMBC correlations from H3-16 to C-1,C-2,and C-3 indicated a location of the methyl group (CH316) at C-2. The gHMBC spectrum of 1 showed two- and three-bond correlations from H3-17 to C-5,C-6,C-7,and C-13. This reveals a connection of the quaternary carbon C-6 to C- 5,C-7,C-13,and C-17. The HMBC correlations from both H3-18 and H3-19 to C-9,C-10,and C-11 and from H-12 to C-9 and C-11, together with their chemical shifts,demonstrated the presence of a 11-aldehyde-10,10-dimethyl cyclopropane ring terminalmoiety in 1. In addition,the 1H-1H gCOSY correlation between H-13 and H3-20 and the HMBC correlations from H3-20 to C-13 and C-14 and from H-13 to C-14 indicated that the carbonyl (C-14) and secondary methyl (CH3-20) groups were attached to C-13,while theHMBC correlation from H320 to C-6 confirmed the connection between C-6 and C-13. The HMBC correlations from H-1b to C-15 and from OH-15 to C-4,C-14,and C-15,combined their chemical shifts,demonstrated that the hydroxylated quaternary carbon C- 15 was linked by C-1,C-4,and C-14 to form 5/6 fused-rings in 1, which commonly occurs in the Euphorbia diterpenoids [11, 12, 13, 14]. Although the carbonyl resonance of the cinnamoyl group was not observed in the NMR spectra of 1 because of limitation of the sample amount,the gHMBC spectrum showed correlations from H-7' to a carbon resonance at δC 168.0 (C-9') and C-2'(C-6'),from H-8' to C-1',and fromH-2'(H-6') to C-4'. This,combined with the chemical shifts of H-3 and C-3 and the molecular composition, verified the presence of a cinnamoyloxy group at C-3 in 1. Therefore,the planer structure of 1 was elucidated as shown in Fig. 2,which possesses a structural feature similar to that of the co-occurring euphoractin G [13] except for a carbon bond cleavage between C-12 and C-13 to afford the new skeleton in 1 (Fig. 3).
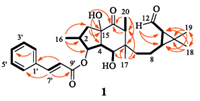
|
Download:
|
| Fig. 2.Main 1H-1H COSY (black thick lines) and HMBC correlations (red arrows,from 1H to 13C) of secoeuphoractin (1). | |
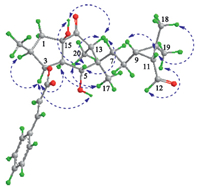
|
Download:
|
| Fig. 3.Main NOE enhancements (blue double arrows) of secoeuphoractin (1). | |
The configuration of 1 was elucidated by analysis of the NOE difference experiment data,together with the CD data and a plausible biogenetic pathway. In the NOE difference spectra, irradiation of H-3 enhanced H-2,H-4,and OH-5. The resonances of H-4 and H320 were enhanced upon irradiation of H317. These enhancements indicate that H-2,H-3,H-4,H317,and H320 are cofacial on the 5/6-fused ring system. Irradiation of H-5 enhanced H-7a,H-13,and OH-15,indicating that H-5,H-7a,H-13,and 15-OH are oriented on the other side of the fused rings. In addition, irradiation of H-11 induced enhancements of H-9 and H318 while H319 was enhanced upon irradiation of H-12. This demonstrates H-9,H-11,and H318 are oriented on one side of the cyclopropane ring whereas H319 and H-12 are on the other side. The CD spectrum of 1 displayed a positive Cotton effect at 301 nm (△ε = +0.77),corresponding to the n-π* transition of the cyclohexanone chromophore. From the octant rule for cyclohexanones [15, 16] (Supporting information) and the aforementioned NOE enhancements,the absolute configuration of the 5/6-fused ring moiety in 1 could therefore be assigned as shown in Fig. 1. A plausible biosynthetic pathway for 1 is postulated in Scheme 1. The biosynthetic precursor of 1 is proposed to be the co-occurring 3- cinnamoyloxy-5,6-epoxylathyr-12-en-15-ol-14-one (2) [12] or/ and euphoractin G (3) [13]. An enzyme-catalyzed transannular cyclization of 2,with a concomitant addition of one molecule of water,generates an intermediate 3,which is supported by the acid-catalyzed transformation of lathyrane analogs [12]. The intermediate would then undergo a sequential and/or simultaneous retro-aldol rearrangement to afford 1. This suggests that the absolute configuration of the cyclopropane ring moiety in 1 is identical to that in the lathyrane analogs and euphoractins,of which the configurations were confirmed by X-ray crystallographic analysis of 3,15-diacetoxy-5,6-epoxylathyr-12-en-14-one [12]. The absolute configuration of 1 was further supported by comparison of the experimental ECD spectrum with that predicted from quantum mechanical time dependent density functional theory (TDDFT) calculations [17]. The theoretically calculated ECD spectrum of 1 was in agreement with the experimental ECD spectrum (Supporting information). Therefore,the structure of compound 1 was determined as shown and designated as secoeuphoractin.
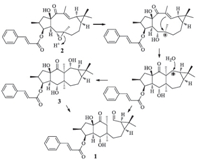
|
Download:
|
| Scheme 1.The plausible biosynthetic pathway of secoeuphoractin (1). | |
In order to exclude the possibility of 1 being fortuitously formed by some type of catalytic effect during the isolation procedure,the putative precursors 2 and 3 were separately refluxed in acetone or methanol with or without silica gel (the main solvents and absorbent used in the isolation procedure) for 48 h. The cyclization and/or ring opening did not occur under the simulated conditions, thus supporting that compound 1 is a true natural product.
In the in vitro bioassay performed in this study,a cell-based VSVG/HIV-1 pseudotyping system for evaluating anti-HIV-1 replication activity [11, 12, 13, 14],compound 1 showed activity against HIV-1 replication with IC50 and SI values of 1.76 ± 0.61 μmol/L and 102,respectively (the positive control zidovudine gave IC50 = 0.05 ± 0.03 μmol/L and SI = 682.2). 4. Conclusion
Secoeuphoractin (1) with activity against HIV-1 replication was isolated as a minor component from the extract of E. micractina roots. Its unique structure,which has never been identified in a natural product,adds a new structural type to the diverse diterpenoids,and provides a new framework for synthesis and biological evaluation. In particular,the plausible biosynthetic pathway associated to the different types of co-occurring diterpenoids provides an important clue for further studies of biomimetic and total synthesis,chemical transformation,structural modification,and structure-activity relationships,as well as biosynthesis of the diverse diterpenoids from the genus Euphoria.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (NNSFC; Nos. 21172266 and 30825044),the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT,No. IRT1007),and the National Science and Technology Project of China (No. 2012ZX09301002-002) are acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.09.012.
| [1] | Z.Y. Wu, T.Y. Zhou, P.G. Xiao, Xin Hua Ben Cao Gang Yao, vol. 2, Shanghai Science and Technology Press, Shanghai, 1991, p. 219. |
| [2] | Q.W. Shi, X.H. Su, H. Kiyota, Chemical and pharmacological research of the plants in genus Euphorbia, Chem. Rev. 108 (2008) 4295-4327. |
| [3] | G.M. Keating, Ingenol mebutate gel 0.015% and 0.05%: in actinic keratosis, Drugs 72 (2012) 2397-2405. |
| [4] | S. Bocklandt, P.M. Blumberg, D.H. Hamer, Activation of latent HIV-1 expression by the potent anti-tumor promoter 12-deoxyphorbol 13-phenylacetate, Antiviral Res. 59 (2003) 89-98. |
| [5] | P. Forgo, D. Redei, Z. Hajdu, et al., Unusual tigliane diterpenes from Euphorbia grandicornis, J. Nat. Prod. 74 (2011) 639-643. |
| [6] | A. Vasas, E. Sulyok, D. Redei, et al., Jatrophane diterpenes from Euphorbia esula as antiproliferative agents and potent chemosensitizers to overcome multidrug resistance, J. Nat. Prod. 74 (2011) 1453-1461. |
| [7] | L.L. Pan, P.L. Fang, X.J. Zhang, et al., Tigliane-type diterpenoid glycosides from Euphorbia fischeriana, J. Nat. Prod. 74 (2011) 1508-1512. |
| [8] | I.S. Aljancic, M. Pesic, S.M. Milosavljevic, et al., Isolation and biological evaluation of jatrophane diterpenoids from Euphorbia dendroides, J. Nat. Prod. 74 (2011) 1613-1620. |
| [9] | J. Xu, Y. Guo, C. Xie, et al., Bioactive myrsinol diterpenoids from the roots of Euphorbia prolifera, J. Nat. Prod. 74 (2011) 2224-2230. |
| [10] | W.X. Song, Y.C. Yang, J.G. Shi, Two new b-hydroxy amino acid-coupled secoiridoids from the flower buds of Lonicera japonica: isolation, structure elucidation, semisynthesis, and biological activities, Chin. Chem. Lett. 25 (2014) 1215-1219. |
| [11] | W. Xu, C. Zhu, W. Cheng, et al., Chemical constituents of the roots of Euphorbia micractina, J. Nat. Prod. 72 (2009) 1620-1626. |
| [12] | Y. Tian, W. Xu, C. Zhu, et al., Lathyrane diterpenoids from the roots of Euphorbia micractina and their biological activities, J. Nat. Prod. 74 (2011) 1221-1229. |
| [13] | Y. Tian, W. Xu, C. Zhu, et al., Diterpenoids with diverse skeletons from the roots of Euphorbia micractina, J. Nat. Prod. 76 (2013) 1039-1046. |
| [14] | Y. Tian, Q. Guo, W. Xu, et al., A Minor Diterpenoid with a new 6/5/7/3 fused-ring skeleton from Euphorbia micractina, Org. Lett. 16 (2014) 3950-3953. |
| [15] | X.L. Ye, Stereochemistry, Beijing University Express, Beijing, 1999. |
| [16] | D.A. Lightner, The octant rule, in: K. Nakanish, N. Berova, R.W. Woody (Eds.), Circular dichroism principles and applications, Wiley-VCH Inc., New York, 1994, pp. 259-299. |
| [17] | X.C. Li, D. Ferreira, Y.Q. Ding, Determination of absolute configuration of natural products: theoretical calculation of electronic circular dichroism as a tool, Curr. Org. Chem. 14 (2010) 1678-1697. |





