Modern organic chemistry is more and more related to heterocycles. Developing novel heterocycles and relating synthetic methods is the eternal goal in synthetic chemistry [1]. Of particular interest is the nitrogen-containing heterocycles due to their existence in many natural or synthetic molecules [2]. Despite diverse synthetic utility,exploring novel synthetic methods to meet increasing scientific and practical demands is still an active area.
Heterocyclic ketene aminals (HKAs) are important versatile intermediates in synthetic chemistry [3]. Moreover,HKAs per se and their derivatives exhibited pharmaceutical [4] or pesticidal activity [5]. Their usage for constructing a variety of nitrogencontaining heterocycles or fused heterocyclic compounds has been extensively studied [6]. The notable feature of HKAs is the highly polarized ethylene systems with electro-donating amino group and electro-withdrawing substituent at two ends (Fig. 1). This unique structural feature makes them serve as bis-nucleophiles reacting with various bis-electrophiles [3].

|
Download:
|
| Fig. 1.Heterocyclic ketene aminals. | |
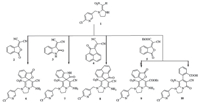
Our interests in HKAs chemistry involved the preparation of bioactive molecules using them and their methodology studies [5, 7]. During previous investigation,we found that HKA 1 could react with cyclic Knoevenagel adducts (KAs) 2,3 and 4 affording the corresponding spiro-heterocyclic compounds 6,7 and 8. However, when the same type of KA 5 was employed,an unknown product instead of spiro-heterocyclic product 9 formed. Further structural analysis proved that this new product was 10 (Fig. 2). The above unexpected results were especially interesting and useful because they provided an novel way for entry to 2-oxo-1,2- dihydropyridine-fused heterocycles. As there were only two reports on the access of this kind of novel heterocycles via azaene reaction,cyclization and oxidation [6h, 6g],it was necessary to develop this novel methodology.
|
Download:
|
| Fig. 2.Reaction of HKAs with cyclic Knoevenagel adducts. | |
In the current study,we wish to report a facile synthesis of 2- oxo-1,2-dihydropyridine-fused 1,3-diazaheterocycles from HKAs, phthalic anhydride and ethyl cyanacetate. Moreover,this method provided the possibility for assembling benzoic acid subunit which can be utilized for further derivatization since the introduction of this substituted pattern is still remaining difficult.
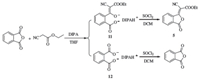
For the purity of KA 5 is of critical importance,initially,its preparation was investigated. The conversion of phthalic anhydride to KA 5 was attempted by a two-step process consisting of the addition of ethyl cyanacetate under diisopropylamine (DIPA) and THF followed by cyclization in SOCl2 and dichloromethane (DCM). In a preliminary experiment,TLC analysis of the reaction mixture indicated that only one product existed in the reaction mixture. After purification,however,ultra performance liquid chromatography (UPLC) and 1H NMR showed that the product was a mixture of 5 and phthalic anhydride (with a ratio of 1:5) which had similar Rf values. Further study elucidated that treatment of phthalic anhydride with DIPA would also lead to the formation of salt 12 which could convert to phthalic anhydride during cyclization. By careful selection of eluting solvents,the pure KA 5 was successfully obtained eluting by DCM/n-hexane (2:1,v:v) (Fig. 3).
|
Download:
|
| Fig. 3.Synthesis of KA 5. | |
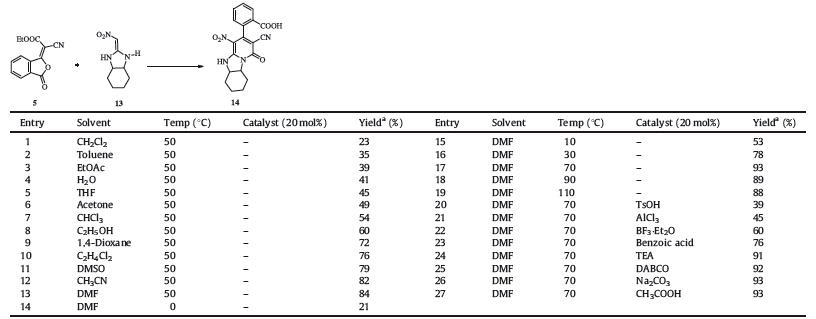
With the pure 5 in hand,we next optimized the reaction conditions for this transformation. As a model reaction, 2-(nitromethylene) octahydro-1H-benzo[d]imidazole 13 and 5 were treated with different solvents at varied temperature (Table 1). A rapid screen of solvents at 50 ℃ without catalysts showed significant reduction of the formation of 14 when the reaction was carried out in nonpolar or low polar solvents (such as THF and toluene). DMF was used for further evaluation due to the high conversion. Elevating temperature to 70 ℃ resulted in the highest yields (93%). When the reaction temperature went above 70 ℃,slight decomposition of 5 was observed. Acid or base did not show any increases in production rate on this transformation.
| Table 1 Optimization of reaction of HKA 13 with 5. |
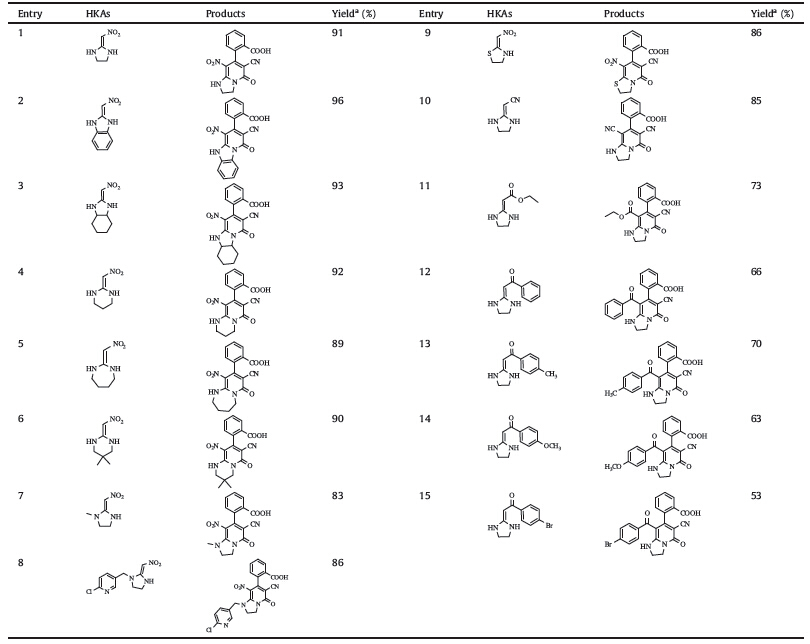
Under the optimal conditions (DMF,70 ℃),a variety of HKAs were examined to probe the generality as well as the limitation of this novel method. The results were summarized in Table 2. This protocol was tolerant to various HKAs. The electron-withdrawing ability ofEWGhad a great influence on the reaction proceeding. For substrates with strong electron-withdrawing group (-NO2 or -CN), the products were obtained in excellent yields (entries 1-10, around 90%). In cases where the EWG was -COOR or -COR,the reactions afforded products in lower yields due to the poorer electron-withdrawing ability (entries 11-15). The cycle patterns and the electronic properties of substituents on nitrogen in HKAs had little effect on this transformation.
| Table 2 Synthesis of 2-oxo-1,2-dihydropyridine-fused 1,3-diazaheterocycles. |
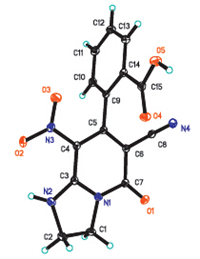
The structures of the products were well characterized by NMR and HRMS studies. Furthermore,diffraction studies allowed for the unambiguous confirmation of the inferred structure (Fig. 4).
|
Download:
|
| Fig. 4.ORTEP drawing of 2-(6-cyano-8-nitro-5-oxo-1,2,3,5-tetrahydroimidazo[1,2- a]pyridin-7-yl)benzoic acid. | |
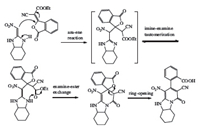
Although the mechanism of reaction was not established experimentally,a possible explanation was proposed here to elucidate this conversion. The transformation proceeded in the following sequential reactions (using HKA 13 as example),aza-ene reaction,imine-enamine tautomerization,enamine-ester exchange and ring-opening reaction (Fig. 5).
|
Download:
|
| Fig. 5.Proposed mechanism for the formation of 2-oxo-1,2-dihydropyridine-fused 1,3-diazaheterocycle. | |
In conclusion,we developed a procedure for the convenient synthesis of 2-oxo-1,2-dihydropyridine-fused 1,3-diazaheterocycles by simply stirring a mixture of knoevenagel adduct formed by phthalic anhydride and ethyl cyanacetate and heterocyclic ketene aminal. This transformation presented a novel method for the construction of 2-oxo-1,2-dihydropyridine-fused 1,3-diazaheterocycles. Moreover,the benzonic acid subunit was simultaneously introduced,which made them easy for further transformation for constructing derivatives with biological importance.
AcknowledgmentsThis work was financial supported by National Natural Science Foundation of China (No. 21372079),National High Technology Research Development Program of China (863 Program,No. 2011AA10A207),Shanghai Pujiang Program (No. 14PJD012),Key Projects in the National Science & Technology Pillar Program (No. 2011BAE06B05),and the Fundamental Research Funds for the Central Universities. This work was also partly supported by Australia DC Foundation.
| [1] | (a) J. Alvarez-Builla, J.J. Vaquero, J. Barluenga, Modern Heterocyclic Chemistry, John Wiley & Sons, New York, 2011; (b) J.A. Joule, K. Mills, Heterocyclic Chemistry at a Glance, Blackwell Pub., Oxford, UK, 2007; (c) A.F. Pozharskii, A.T. Soldatenkov, A.R. Katritzky, Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry and Biochemistry and the Role of Heterocycles in Science, Technology, Medicine and Agriculture, John Wiley & Sons, New York, 1997. |
| [2] | H. El Sayed, A. El Nemr, Synthesis of Naturally Occurring Nitrogen Heterocycles from Carbohydrates, John Wiley & Sons, New York, 2008. |
| [3] | (a) S.J. Yan, Y.F. Niu, R. Huang, J. Lin, Synthesis of bicyclic pyridones via cyclocondensation of heterocyclic ketene aminals with β-Ketoester enol tosylates, Synlett (2009) 2821-2824; (b) Y. Muhammd, C.Y. Yu, Y.M. Jia, Z.T. Huang, Reactions of heterocyclic ketene aminals with Baylis-Hillman acetates: a novel synthesis of tetrahydropyridinefused 1,3-diazaheterocycles, Synlett 9 (2008) 1357-1360; (c) M. Li, Z.M. Zhou, L.R. Wen, Z.X. Qiu, Chemistry of heterocyclic ketene aminals: construction of imidazo(pyrido)[1,2-a]pyridines and imidazo(pyrido)[3,2,1-ij][1,8]-naphthyridines via DABCO-catalyzed tandem annulations, J. Org. Chem. 76 (2011) 3054-3063; (d) L.R. Wen, C. Liu, M. Li, L.J. Wang, Modulating the reactivity of heterocyclic ketene aminals in MCR: selective construction of tetrahydrobenzo[b]imidazo[3,2,1-ij][1,8]naphthyridines, J. Org. Chem. 75 (2010) 7605-7614. |
| [4] | (a) M.C. Saada, J.L. Montero, D. Vullo, et al., Carbonic anhydrase activators: gold nanoparticles coated with derivatized histamine, histidine, and carnosine show enhanced activatory effects on several mammalian isoforms, Eur J. Med. Chem. 54 (2011) 1170-1177; (b) H. Kondo, M. Taguchi, Y. Inoue, F. Sakamoto, G. Tsukamoto, Synthesis and antibacterial activity of thiazolo-, oxazolo-, and imidazolo[3,2-a][1,8]napht hyridinecarboxylic acids, J. Med. Chem. 33 (1990) 2012-2015; (c) M.M. Abdelhalim, M.T. El-Saidi, S.T. Rabie, G.A. Elmegeed, Synthesis of novel steroidal heterocyclic derivatives as antibacterial agents, Steroids 72 (2007) 459-465; (d) B.E. Maryanoff, W. Ho, D.F. McComsey, et al., Potential anxiolytic agents. Pyrido[1,2-a]benzimidazoles: a new structural class of ligands for the benzodiazepine binding site on GABA-A receptors sit on GABA-A receptors, J. Med. Chem. 38 (1995) 16-20; (e) S.J. Yan, Y.J. Liu, Y.L. Chen, L. Liu, J. Liu, An efficient one-pot synthesis of heterocycle-fused 1,2,3-triazole derivatives as anti-cancer agents, Bioorg. Med. Chem. Lett. 20 (2010) 5225-5228; (f) B.F. Jensen, C. Vind, S.B. Padkjaer, P.B. Brockhoff, In silico prediction of cytochrome P450 2D6 and 3A4 inhibition using Gaussian kernel weighted knearest neighbor and extended connectivity fingerprints, including structural fragment analysis of inhibitors versus noninhibitors, J. Med. Chem. 50 (2007) 501-511; (g) X.S.Shao,H.Fu,X.Y.Xu,etal.,Divalentandoxabridgedneonicotinoids constructed by dialdehydes and nitromethylene analogues of imidacloprid: design, synthesis, crystal structureandinsecticidalactivities, J.Agric.Food.Chem.58(2010)2696-2702. |
| [5] | (a) Z.Z. Tian, X.X. Shao, Z. Li, X.H. Qian, Q.C. Huang, Synthesis, insecticidal activity, and QSAR of novel nitromethylene neonicotinoids with tetrahydropyridine fixed cis configuration and exo-ring ether modification, J. Agric. Food Chem. 55 (2007) 2288-2292; (b) X.S. Shao, Z.P. Xu, X.F. Zhao, et al., Synthesis, crystal structure and insecticidal activities of highly congested hexahydroimidazo[1,2-a]pyridine derivatives: effect of conformation on activities, J. Agric. Food Chem. 58 (2010) 2690-2695; (c) X.S. Shao, P.W. Lee, Z.W. Liu, et al., cis-Configuration: a new tactic/rationale for neonicotinoid molecular design, J. Agric. Food Chem. 59 (2011) 2943-2949; (d) K. Baum, S.S. Bigelow, N.V. Nauyen, et al., Synthesis and reactions of 1,1-diiododinitroethylene, J. Org. Chem. 57 (1992) 235-241. |
| [6] | (a) C.C. Zeng, F.J. Liu, D.W. Ping, et al., One-pot electrochemical synthesis of fused indole derivatives containing active hydroxyl groups in aqueous medium, J. Org. Chem. 74 (2009) 6386-6389; (b) C.Y. Yu, P.H. Yang, M.X. Zhao, Z.T. Huang, A novel one-pot reaction of heterocyclic ketene aminals: synthesis of a small library of tetrahydropyridinone-fused 1,3-diazaheterocycles, Synlett 12 (2006) 1835-1840; (c) M.X. Wang, Z.T. Huang, Regiospecific allylation of benzoyl-substituted heterocyclic ketene aminals and their zinc chloride-promoted 3-aza-cope rearrangement, J. Org. Chem. 60 (1995) 2807-2811; (d) Z.T. Huang, M.X. Wang, The reaction of benzoyl-substituted heterocyclic ketene aminals with aryl azides, J. Org. Chem. 57 (1992) 184-190; (e) Q. Yang, Z.J. Li, X.M. Chen, Z.T. Huang, The reaction of heteroaroyl-substituted heterocyclic ketene aminals with 2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl azide, Heteroat. Chem. 13 (2002) 242-247; (f) J.P. Liao, T. Zhang, C.Y. Yu, Z.T. Huang, Reaction of heterocyclic ketene aminals with bis(methylthio)methylene malononitrile: synthesis of polyfunctionalized pyridine-fused 1,3-diazaheterocycles from heterocyclic ketene aminals, Synlett 5 (2007) 0761-0764;(g) L.R. Wen, C.Y. Jiang, M. Li, L.J. Wang, Application of 2-(2-chloroaroyl)methyleneimidazolidines in domino and multicomponent reaction: new entries to imidazo[1,2-a]pyridines and benzo[b]imidazo[1,2,3-ij][1,8]naphthyridines, Tetrahedron 67 (2011) 293-302; (h) A. Alizadeh, A. Rezvanian, A novel and efficient synthesis of pyrido[1,2-a]-fused 1,3-diazaheterocyclic compounds via a one-pot three-component reaction, Helvetica Chim. Acta 95 (2012) 152-156; (i) A. Alizadeh, J. Mokhtari, M. Ahmadi, Synthesis of the novel pyrimido[1,6-a]pyrimidine and imidazo[1,2-c]pyrimidine derivatives based on heterocyclic ketene aminals, Tetrahedron 68 (2012) 319-322; (j) A. Alizadeh, N. Zohreh, A unique approach to catalyst-free, one-pot synthesis of spirooxindole-pyrazolines, Synlett 23 (2012) 428-432; (k) V.J. Ram, N. Agarwal, A. Sharon, P.R. Maulik, An expeditious synthesis of imidazo[1,2-a]pyridines through nucleophile induced ring transformation reactions of 6-aryl-4-methylsulfanyl-2H-pyran-2-one-3-carbonitriles, Tetrahedron Lett. 43 (2002) 307-310; (l) W.Y. Xu, Y.M. Jia, J.K. Yang, Z.T. Huang, C.Y. Yu, Reactions of heterocyclic ketene aminals with 2-[3-oxoisobenzofuran-1(3H)-ylidene]malononitrile: synthesis of novel polyfunctionalized 1,4-dihydropyridine-fused 1,3-diazaheterocycles, Synlett 11 (2010) 1682-1684; (m) S. Chakrabarti, K. Panda, N.C. Misra, H. Ila, H. Junjappa, Aza-annulation of polarized N,S-and N,N-ketene acetals with itaconic anhydride: synthesis of novel functionalized 1,2,3,4-tetrahydro-2-pyridones and related azabicycles, Synlett 9 (2005) 1437-1441; (n) L.P. Ren, Y.P. Lou, N.Y. Chen, et al., Synthetic communications: an international journal for rapid communication of synthetic organic chemistry, Synth. Commun. 44 (2013) 858-867; (o) R.C.F. Jones, P. Patel, S.L. Hirst, M.J. Smallridge, Annulation of imidazolines with bis-electrophiles: synthesis of imidazo[1,2-a]pyridines, Tetrahedron 54 (1998) 6191-6200; (p) D. Sucunza, A. Samadi, M. Chioua, et al., A practical two-step synthesis of imidazo[1,2-a]pyridines from N-(prop-2-yn-1-yl)pyridin-2-amines, Chem. Commun. 47 (2011) 5043-5045. |
| [7] | (a) Z.J. Ye, L.N. Shi, X.S. Shao, et al., Pyrrole-and dihydropyrrole-fused neonicotinoids: design, synthesis, and insecticidal evaluation, J. Agric. Food Chem. 61 (2013) 312-319; (b) S.Y. Lu, X.S. Shao, Z. Li, et al., Design, synthesis, and particular biological behaviors of chain-opening nitromethylene neonicotinoids with cis configuration, J. Agric. Food. Chem. 60 (2012) 322-330; (c) B.Z. Wang, J.G. Chen, Z.P. Xu, et al., Synthesis and biological activity evaluation of novel (-substituted nitromethylene neonicotinoid analogues, Molecules 17 (2012) 10014-10025; (d) X.S. Shao, Z.J. Ye, H.B. Bao, et al., Advanced research on cis-neonicotinoids, Chimia 65 (2011) 957-960; (e) Z.J. Ye, S. Xia, X.S. Shao, et al., Design, synthesis, crystal structure analysis, and insecticidal evaluation of phenylazoneonicotinoids, J. Agric. Food Chem. 59 (2011) 10615-10623; (f) R.B. Xu, R. Xia, M. Luo, et al., Design, synthesis, crystal structures, and insecticidal activities of eight-membered azabridge neonicotinoid analogues, J. Agric. Food Chem. 62 (2014) 381-390; (g) N.Y. Chen, L.P. Ren, M.M. Zou, et al., Design, synthesis and insecticidal activity of spiro heterocycle containing neonicotinoid analogs, Chin. Chem. Lett. 25 (2014) 197-200. |