Multicomponent reactions (MCRs) are economically and environmentally advantageous as three or more starting materials can be reacted in a one-pot procedure to give a single product [1,2,3 ].
The quinoxaline nucleus is present in many biologically and pharmaceutically active compounds. Quinoxalines show antiinflammatory [4],antiviral [5],antiglucoma [6],herbicidal [7],and anticancer [8] activities. Furthermore,the synthesis of quinoxalines and their derivatives has received much attention from organic and medicinal chemists. Nevertheless,most of the reported methods for the synthesis of pyrrolo[1,2-a]quinoxalines suffer from one or more disadvantages which limit their use,such as: difficulties in product isolation,the use of highly expensive and detrimental metal precursors,unsatisfactory yields,and longer reaction times [9, 10, 11, 12].
Iron(Ⅲ) chloride has been used as an efficient catalyst for the manufacture of carbon-heteroatom and heteroatom-heteroatom bonds with considerable advantages [13]. It has been used previously by us for the synthesis of pyrrolo [2,1-c]benzoxazines [14]. As part of our current studies on the development of new routes to synthesize heterocyclic systems [15, 16, 17],the syntheses of pyrrolo[1,2-a]quinoxaline and pyrrolo[1,2-a] pyrazine derivatives from 1,2-diamines (1),ethyl pyruvate (2)anda-bromo ketones (3) in the presence of FeCl3as a catalyst are reported herein (Scheme 1).

|
Download:
|
| Scheme 1.Three-component synthesis of pyrrolo[1,2-a]quinoxaline and pyrrolo[1,2-a]pyrazine derivatives | |
Melting point (mp) was measured on a microscopic melting point apparatus. The IR spectra were recorded on a Shimadzu 460 FT-IR spectrometer with a KBr disk. 1 H NMR and 13 C NMR spectra were taken on a Bruker DRX-250 Avance spectrometer at 250 MHz and 62.5 MHz in DMSO-d6,chemical shift are given in part per million (ppm) relative to TMS as an internal standard. Mass spectra and high resolution mass spectra were performed on FinniganMAT-8430 mass spectrometer with electron spray ionization (ESI) as the ionization mode. Elemental analyses were obtained using Heraeus CHN-O-Rapid analyzer.
Typical procedure for the preparation of compounds4a-h, exemplified by 4a: In a round-bottom flask equipped with a magnetic stirrer,1,2-phenylenediamine (2 mmol) and ethyl pyruvate (2 mmol) in MeCN (3 mL) were added,and the mixture was stirred vigorously at room temperature. Ethyl bromopyruvate (2 mmol) in MeCN (2 mL) and FeCl3(20 mol%) were added to the mixture,which was refluxed for 5 h. After completion of the reaction,as indicated by TLC (EtOAC/hexane = 1/3,v/v),the mixture was cooled to room temperature. The solvent was evaporated and the residue was purified by column chromatography usingn-hexane/EtOAc (3/1,v/v) as the eluent. The solvent was removed and the product was obtained.
Ethyl 4-oxo-4,5-dihydropyrrolo[1,2-a]quinoxaline-2-carboxylate (4a): Yield (0.20 g,78%),grey crystals; mp 240-2428℃. IR (KBr, cm -1 ): vmax3419 (NH),1721 (C55O),1669 (C55O). 1 H NMR (250.1 MHz,DMSO-d6):δ1.30 (t,3H, 3 J= 7.0 Hz,CH3),4.27 (q,2H, 3 J= 7.0 Hz,OCH2),7.19-7.35 (m,4H,4CH),8.22 (d,1H, 3 J= 8.0 Hz, CH),8.75 (s,1H,CH),11.45 (br s,1H,NH). 13 C NMR (62.9 MHz, DMSO-d6):δ14.7 (CH3),60.5 (OCH2),111.9 (CH),116.3 (CH),117.1 (CH),119.4 (C),121.8 (CH),122.5 (C),123.4 (CH),124.7 (C),127.3 (C),129.4 (CH),155.2 (C55O),163.6 (C55O). MS:m/z(%): 256 (M + , 100),241 (8),228 (44),211 (100),183 (47),155 (42),77 (15). Anal. Calcd. for C14H12N2O3(256.26): C,65.62; H,4.72; N,10.93; found: C,65.57; H,4.69; N,10.96.
2-Phenylpyrrolo[1,2-a]quinoxaline-4(5H)-one (4b): Yield (0.18 g, 70%),yellow oil. IR (KBr,cm -1 ):vmax3423 (NH),1662 (C55O). 1 H NMR (250.1 MHz,DMSO-d6):δ7.21-7.53 (m,9H,9CH),8.12 (d,1H, 3 J= 8.0 Hz,CH),8.62 (s,1H,CH),11.47 (br s,1H,NH). 13 CNMR (62.9 MHz,DMSO-d6):δ111.9 (CH),116.0 (C),117.1 (CH),117.2 (C), 121.6 (C),122.2 (CH),123.4 (CH),126.5 (CH),126.9 (CH),127.3 (2CH),127.8 (CH),129.0 (C),129.3 (2CH),133.1 (C),154.3 (C55O). MS: m/z(%): 260 (M+ ,100),232 (13),218 (24),183 (86),77 (35). Anal. Calcd. for C17H12N2O (260.30): C,78.44; H,4.65; N,10.76; found: C,78.57; H,4.61; N,10.79.
2-(4-Bromophenyl)pyrrolo[1,2-a]quinoxalin-4(5H)-one (4c): Yield (0.25 g,73%),pink crystals; mp 119-1218℃. IR (KBr, cm -1 ): vmax 3315 (NH),1667 (C55O). 1 H NMR (250.1 MHz, DMSO-d6): δ 7.17-7.48 (m,8H,8CH),8.09 (d,1H, 3J= 8.1 Hz, CH),8.65 (s,1H,CH),11.42 (br s,1H,NH). 13CNMR (62.9 MHz, DMSO-d6):d116.8 (CH),118.2 (CH),123.1 (C),123.8 (CH),124.4 (CH),125.1 (CH),125.8 (2CH),126.3 (C),129.8 (C),132.2 (2CH), 132.9 (C),135.4 (CH),136.6 (C),139.2 (C),154.1 (C55O). MS:m/z (%): 339 (M+ ,9),297 (28),218 (91),183 (96),157 (38),76 (62),57 (100). Anal. Calcd. for C17H11BrN2O (339.19): C,60.20; H,3.27; N, 8.26; found: C,60.27; H,3.21; N,8.32.
1-Methyl-2-p-tolylpyrrolo[1,2-a]quinoxalin-4(5H)-one (4d): Yield (0.20 g,72%),yellow powder; mp 60-618C. IR (KBr,cm -1 ): vmax3200 (NH),1663 (C55O). 1 H NMR (250.1 MHz,DMSO-d6): δ 2.29 (s,3H,CH3),7.21-7.39 (m,5H,5CH),7.64-7.67 (m,3H,3CH), 8.07 (s,1H,CH),8.64 (s,1H,CH),11.23 (br s,1H,NH). 13CNMR (62.9 MHz,DMSO-d6):d22.3 (CH3),116.1 (CH),116.6 (CH),118.1 (CH),124.0 (C),124.4 (CH),125.8 (CH),126.9 (2CH),127.3 (C), 129.4 (C),130.1 (CH),130.5 (C),131.0 (2CH),132.5 (C),137.5 (C), 156.5 (C55O). MS:m/z(%): 274 (M+ ,100),246 (6),232 (41),183 (81),91 (32),76 (18). Anal. Calcd. for C18H14N2O (274.33): C,78.81; H,5.14; N,7.63; found: C,78.94; H,5.19; N,7.69.
Ethyl 7,8-diamethy1-4-oxo-4,5-dihydroptrrolo[1,2-a]quinoxaline-1-carboxylate (4e): Yield (0.24 g,84%),grey crystals; mp 237- 2398℃. IR (KBr,cm -1 ):vmax3305 (NH),1731 (C55O),1623 (C55O). 1 H NMR (250.1 MHz,DMSO-d6):δ1.23 (t,3H, 3J= 7.1 Hz,CH3),2.24 (s,3H,CH3),2.47 (s,3H,CH3),4.27 (q,2H, 3J= 7.0 Hz,OCH2),7.08 (s, 1H,CH),7.32 (s,1H,CH),7.75 (s,1H,CH),8.33 (s,1H,CH),11.45 (br s,1H,NH). 13CNMR (62.9 MHz,DMSO-d6):δ14.3 (CH3),21.2 (CH3), 22.4 (CH3),61.8 (OCH2),115.6 (CH),118.5 (C),121.0 (CH),123.8 (CH),125.2 (C),128.3 (C),129.4 (CH),133.6 (C),135.3 (CH),136.2 (CH),155.2 (C55O),162.4 (C55O). MS:m/z(%): 284 (M+ ,100),269 (10),256 (45),239 (100),211 (45),183 (38),169 (25). Anal. Calcd. for C14H16N2O3(284.32): C,67.59; H,5.67; N,9.85; found: C,67.62; H,5.69; N,9.81.
2-(4-Bromophenyl)-7,8-dimethylpyrrolo[1,2-a]quinoxalin-4(5H)-one (4f): Yield (0.27 g,74%),grey crystals; mp 235-2378℃. IR (KBr,cm -1 ):vmax3335 (NH),1653 (C55O). 1 H NMR (250.1 MHz, DMSO-d6):δ2.29 (s,3H,CH3),2.38 (s,3H,CH3),6.85(s,1H,CH), 7.23-7.39 (m,3H,3CH),7.48-7.59 (m,2H,2CH),7.71-7.75 (m,1H, CH),8.22 (s,1H,CH),11.45 (br s,1H,NH). 13CNMR (62.9 MHz, DMSO-d6):δ22.3 (CH3),22.8 (CH3),109.9 (CH),116.4 (CH),117.1 (C),118.6 (CH),122.8 (C),124.1 (C),124.3 (C),126.1 (2CH),127.2 (CH),129.3 (C),132.9 (C),130.0 (C),131.1 (2CH),136.4 (C),155.6 (C55O). MS:m/z(%): 367 (M+ ,12),325 (28),246 (94),76 (63),57 (100). Anal. Calcd. for C19CH15BrN2O (367.25): C,62.14; H,4.12; N, 7.63; found: C,62.11; H,4.15; N,7.59.
Ethyl 4-oxo-4,5,5a,6,7,8,9,9a-octahydropyrrolo[1,2-a]quinoxalin-1-carboxylate (4g): Yield (0.18 g,68%),yellow oil. IR (KBr, cm -1 ): vmax3280 (NH),1725 (C55O),1656 (C55O). 1 H NMR (250.1 MHz,DMSO-d6):δ1.22-1.46 (m,7H,2CH2 and CH3),1.75- 1.94 (m,4H,2CH2),3.38-3.54 (m,2H,2CH),4.23 (q,2H, 3J= 7.1 Hz, OCH2),6.76 (d,1H, 4 J= 1.5 Hz,CH),7.69 (d,1H, 4 J= 1.5 Hz,CH), 11.28 (1H,br s,NH). 13CNMR (62.9 MHz,DMSO-d6):δ14.3 (CH3), 23.2 (CH2),24.8 (CH2),31.2 (CH2),31.8 (CH2),54.7 (CH),60.5 (CH2), 61.2 (OCH2),111.3 (C),118.6 (CH),126.3 (C),132.2 (CH) 158.4, 164.7 (2 C55O). MS:m/z(%): 262 (M+ ,100),247 (15),217 (51),189 (100),161 (39). Anal. Calcd. for C14H18N2O3(262.31): C,64.11; H, 6.92; N,10.68; found: C,64.05; H,6.88; N,10.76.
Ethyl 1-oxo-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine-7-carboxylate (4h): Yield (0.15 g,72%),yellow crystals; mp 236-2388℃. IR (KBr,cm -1 ): vmax3330 (NH),1727 (C55O),1647 (C55O). 1 HNMR (250.1 MHz,DMSO-d6): δ1.27 (t,3H, 3J= 7.1 Hz,CH3),3.46-3.51 (m,2H,CH2),4.11-4.13 (m,2H,CH2),4.21 (q,2H, 3J= 7.0 Hz,OCH2), 6.90 (d,1H, 4 J= 1.4 Hz,CH),7.62 (d,1H, 4 J= 1.4 Hz,CH),11.21 (s,1H, NH). 13CNMR (62.9 MHz,DMSO-d6):δ14.8 (CH3),43.1 (CH2),43.9 (CH2),60.9 (OCH2),114.2 (CH),116.7 (C),118.3 (C),134.6 (CH),156.0 (C55O),163.7 (C55O). MS:m/z(%): 208 (M+ ,100),193 (82),180 (27), 163 (100),135 (20),120 (57),107 (26),77 (27). Anal. Calcd. for C10H12N2O3(208.22): C,57.69; H,5.8; N,13.45; found: C,57.73; H,5.78; N,13.41. 3. Results and discussion
The reaction of 1,2-phenylenediamine,ethyl pyruvate,and ethyl bromopyruvate in the presence of FeCl3 (20 mol%) was selected as a model system (Scheme 2).

|
Download:
|
| Scheme 1.The synthetic routes to the NDAZO and PANDAZO. | |
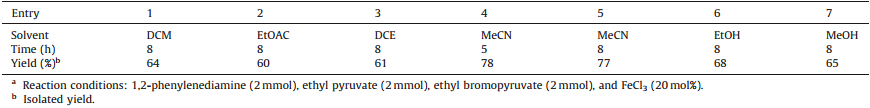
Initially,we thought of varying the nature of solvent to increase the product yield,so we carried out the reactions in dichloromethane,dichloroethane,ethanol,ethyl acetate,acetonitrile,and methanol at reflux temperature (Table 1). When the reaction mixture was refluxed for 5 h in acetonitrile,the yield of 4a was improved significantly (78%). Next the catalytic amount of the Iron(Ⅲ) chloride catalyst was examined in the model reaction. In the presence of 10,15,20,and 25 mol% of FeCl3,the yields of pyrrolo[1,2-a]quinoxaline 4a obtained were 42%,55%,78%,and 78%,respectively. This shows the important role of FeCl3in this reaction. Thus,to verify that this is generally the case,we optimized the conditions for the other reactions. The results are presented in Table 2.
| Table 1 The effect of solvent on the reaction time and yield. a |
| Table 2 Synthesis of pyrrolo[1,2-a]quinoxaline and pyrrolo[1,2-a]pyrazine derivatives 4 |
The 1 H NMR, 13CNMR,IR spectra and MS of the products clearly indicated the formation of compounds 4a-h. For example,the 1 H NMR spectrum of4a exhibited a triplet at 1.21 ppm and a quartet at 4.20 ppm for the ethoxy group,along with multiplets (7.19-8.75 ppm) for the aromatic region,and a broad singlet at 11.45 ppm due to the NH group. The 1 H-decoupled 13CNMR spectrum of 4a showed 14 distinct resonances in agreement with the proposed structure. The IR spectrum of4aexhibited absorption bands due to carbonyl groups at 1721 and 1669 cm -1 . The 1 H NMR and 13CNMR spectra of products 4b-h were similar to those of 4a,except for the ester moieties,which exhibited characteristic resonances in the appropriate parts of the spectrum. On the basis of these results,a possible mechanism for the formation of pyrrolo[1,2-a]quinoxaline 4a is shown in Scheme 3. The reaction between 1,2-diaminobenzene (1a) and ethyl pyruvate (2) affords quinoxaline 5,then ethyl bromopyruvate (3) could be activated by FeCl3 and undergo the nucleophilic addition. The subsequent intermediate 6 by the elimination of the HBr leads to intermediate 7,which undergoes a series of cyclization and elimination reactions to generate product 4a. 4. Conclusion
In conclusion,we have described a simple and efficient method for the synthesis of pyrrolo[1,2-a]quinoxaline and pyrrolo[1,2-a]pyrazine derivatives of potential synthetic and pharmacological interest. This method is characterized by several unique advantages,such as simplicity in operation under neutral conditions, high yields of products,and relatively short reaction time. Acknowledgment
We are grateful to Sanandaj Branch,Islamic Azad University Research Council for the financial support of this research.
| [1] | L. Weber, Multi-component reactions and evolutionary chemistry, Drug Discov. Today 7 (2002) 143-147. |
| [2] | G. Balme, E. Bossharth, N. Monteiro, Pd-assisted multicomponent synthesis of heterocycles, Eur. J. Org. Chem. 21 (2003) 4101-4111. |
| [3] | J. Zhu, H. Bienaymé , Multicomponent Reactions, Wiley-VCH, Weinheim, 2005. |
| [4] | R.A. Smits, H.D. Lim, A. Hanzer, et al., Fragment based design of new H4 receptor- ligands with anti-inflammatory properties in vivo, J. Med. Chem. 51 (2008) 2457- 2467. |
| [5] | A. Furlan, F. Colombo, A. Kover, et al., Identification of new aminoacid amides containing the imidazo[2,1-b]benzothiazol-2-ylphenyl moiety as inhibitors of tumorigenesis by oncogenic Met signaling, Eur. J. Med. Chem. 47 (2012) 239-254. |
| [6] | R. David, Changing therapeutic paradigms in glaucoma management, Exp. Opin. Invest. Drug 7 (1998) 1063-1086. |
| [7] | F. Colombo, C. Tintori, A. Furlan, et al., ‘Click' synthesis of a triazole-based inhibitor of Met functions in cancer cells, Bioorg. Med. Chem. Lett. 22 (2012) 4693-4696. |
| [8] | M.A. Naylor, M.A. Stephen, J. Nolan, et al., Heterocyclic mono-N-oxides with potential applications as bioreductive anti-tumour drugs: Part 1. 8-Alkylamino- substitued phenylimidazo[1,2-a]quinoxalines, Anticancer Drug Res. 8 (1993) 439-461. |
| [9] | C.S. Yi, S.Y. Yun, Scope and mechanistic study of the ruthenium-catalyzed ortho- C-H bond activation and cyclization reactions of arylamines with terminal alkynes, J. Am. Chem. Soc. 127 (2005) 17000-17006. |
| [10] | R. Abonia, B. Insuasty, J. Quiroga, H. Kolshorn, H. Meier, A versatile synthesis of 4,5-dihydropyrrolo[1,2-a]quinoxalines, J. Heterocycl. Chem. 38 (2001) 671-674. |
| [11] | N.T. Patil, R.D. Kavthe, V.S. Raut, V.V.N. Reddy, Platinum-catalyzed formal Markownikoff's hydroamination/hydroarylation cascade of terminal alkynes assisted by tethered hydroxyl groups, J. Org. Chem. 74 (2009) 6315-6318. |
| [12] | N.T. Patil, P.G.V.V. Lakshmi, V. Singh, Au(I)-catalyzed direct hydroamination- hydroarylation and double hydroamination reactions of terminal alkynes, Eur. J. Org. Chem. 24 (2010) 4719-4731. |
| [13] | A. Correa, O.G. Mancheno, C. Bolm, Iron-catalyzed carbon-heteroatom and heteroatom-heteroatom bond forming processes, Chem. Soc. Rev. 37 (2008) 1108-1117. |
| [14] | M. Piltan, L. Moradi, S.A. Zarei, H. Rostami, One-pot multicomponent synthesis of novel tricyclic pyrrolo[2,1-c][1,4]benzoxazines, Chin. Chem. Lett. 25 (2014) 234-236. |
| [15] | I. Yavari, M. Piltan, L. Moradi, Synthesis of pyrrolo[2,1-a]isoquinolines from activated acetylenes, benzoylnitromethanes, and isoquinoline, Tetrahedron 65 (2009) 2067-2071. |
| [16] | M. Piltan, L. Moradi, G. Abasi, S.A. Zarei, A one-pot catalyst-free synthesis of functionalized pyrrolo[1,2-a]quinoxaline derivatives from benzene-1,2-diamine, acetylenedicarboxylates and ethyl bromopyruvate, Beilstein J. Org. Chem. 9 (2013) 510-515. |
| [17] | L. Moradi, M. Piltan, H. Rostami, G. Abasi, One-pot synthesis of pyrrolo[1,2-a]pyrazines via three component reaction of ethylenediamine, acetylenic esters and nitrostyrene derivatives, Chin. Chem. Lett. 24 (2013) 740-742. |