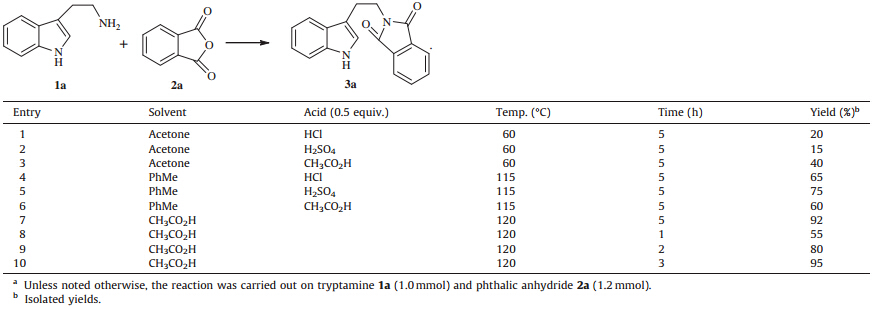
Terpenoid indole alkaloids with a tetrahydro-b-carbolines subunit have been isolated from various plants and animals. The structural diversity of these complex natural products accounts for their plethora of biological activities (Fig. 1) [1, 2, 3]. For instance, tetrahydro-b-carbolines are interesting hypnotic,anxiolytic, antimicrobial,antiviral,anti-tumor,and anticonvulsant compounds. Their broad spectrum of biological and pharmacological activities has attracted considerable interest in their synthesis. In general,the assemblage of this heterocyclic system employs Pictet-Spengler reaction [4],Bischler-Napieralski reaction [5],Nacyliminium ion cyclization [6],and keteniminium Pictet-Spengler reaction [7]. To date,the Pictet-Spengler reaction has proven to be the most straightforward route to synthesize tetrahydro-bcarbolines.
|
Download:
|
| Fig. 1. Selected tetrahydro-b-carboline natural products. | |
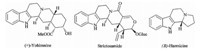
The monoterpenoid indole alkaloid strictosamide,an important biosynthetic precursor of camptothecin,has been isolated from some Nauclea species [8],Psychotria species,and camptothecin producing plants [9, 10]. It is considered to be the main constituent ofNauclea pobeguinii(Rubiaceae) which is active against uncomplicated falciparum malaria as shown in previous clinical studies. In 2012,Ramanathan and his co-workers demonstrated that Brønsted acids can assist 6-exo-trigcyclization of imide derivatives of tryptamine through imide carbonyl activation to assemble the tetrahydro-b-carboline derivatives with strictosamide skeleton [11]. Later,You [12] reported an efficient method to synthesize enantioenriched tetrahydro-b-carbolines with strictosamide skeleton via chiral phosphoric acid catalyzed asymmetric transfer hydrogenation with a Hantzsch ester as the organic hydride source. In this paper,we would like to describe a short,practical,and efficient way to synthesize tetrahydro-b-carbolines with strictosamide skeleton via intermolecular condensation,selective reduction,and intramolecular cyclization from phthalic anhydride and tryptamine (Scheme 1). The reactions were carried out under mild conditions with a wide range of substrates and functional groups.
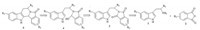
|
Download:
|
| Scheme 1.Retro-synthetic analysis of tetrahydro-b-carbolines. | |
General procedure for the synthesis of5a-5j: A mixture of tryptamine 1a(10.0 mmol) and phthalic anhydride 2a(12.0 mmol) in CH3CO2H (100 mL) was heated to reflux for 3 h (monitored by TLC). After the completion of the reaction,the mixture was left at room temperature,and the solid precipitate was collected by filtration. The precipitate (3a) was rinsed with methanol and used for next step without further purification. The 3a was dissolved in CH2Cl2-CH3OH (2:1,100 mL),and NaBH4(0.45 g,12 mmol) was added slowly at °C with stirring. When the point of 3a disappeared in TLC,6 mol/L HCl (5 mL) was added to the reaction mixture with stirring for 2 h at room temperature. After the reaction was complete,the solvent was evaporated under reduced pressure to give the crude product. The residue was rinsed with methanol and then recrystallized to afford the analytically pure product. A part of the desired products was purified by column chromatography on silica gel with petroleum ether/ethyl acetate as eluent. All the compounds were characterized,and the spectral data was listed in Supporting information. 3. Results and discussion
Initially,different conditions for intermolecular condensation of tryptamine 1a and phthalic anhydride 2a were screened (Table 1). To our delight,the expected phthalimide derivative3a was formed in 90% yield in the presence of CH3CO2H as the catalyst and solvent (Table 1,entries 1-7). The optimization of intermolecular condensation was investigated (Table 1,entries 7-10). At the optimal conditions,the yield was improved to 95%.
| Table 1 Optimization of the intermolecular condensation conditions. a |
The various tryptamine derivatives1and phthalic anhydride derivatives2were tested to examine the generality of the current synthetic route. The results are shown in Fig. 2. The isomers ofb, 5d,and 5fwere not further isolated at this moment due to the very similar polarities. Both the substituents of F and CO2H groups on tryptamine,and NO2 group on the aromatic ring of phthalic anhydride afforded the desired corresponding tetrahydro-bcarboline derivatives 5b-f with 20%-65% yields over three steps. However,with the functionalities of F and CO2H on tryptamine,the yields of intermolecular condensation decreased significantly.
|
Download:
|
| Fig. 2. Structures and isolated yields of synthesized tetrahydro-b-carboline derivatives. ( a Isolated yields of the isomers mixture over 3 steps.) | |
At the optimal conditions,the scope of anhydride was expanded. Starting from tryptamine derivatives1 and succinic anhydride,tetrahydro-b-carboline derivatives (5g-h) were successful synthesized in 46%-72% yields over three steps. The natural productR-harmicine,isolated fromKopsia griffithii,was effectively furnished by the precursor5gusing LiAlH4as a reducing agent in 78% yield [11]. Our methodology made the synthesis of indole alkaloids with further structural complexity easier. Therefore the aliphatic anhydride was also suitable for this sequence of reactions, and the methodology could be used to synthesize tetrahydro-bcarboline derivatives from aliphatic anhydride and tryptamine derivatives. The synthetic efficiency of this methodology was further exemplified through the synthesis of tetrahydro-b-carboline derivatives (5i-j) from tryptamine derivatives 1 and 2,3-pyridinedicarboxylic anhydride. The isomers of5iand 5jwere not further isolated at this moment due to the very similar polarities. A wide substrate scope for this synthetic strategy can be further explored from tryptamine derivatives and pyridinedicarboxylic anhydride.
WhenL-tryptophan (S)-2bwas used as a starting material, according to the mechanism of Pictet-Spengler reaction,the reaction proceeded with a high diastereoselectivity,and the major diastereomer hadcisrelative stereochemistry [13]. Therefore,it was considered that compounds5c,5d,5hand 5jwerecisconfiguration. This deduction was supported by the X-ray structure analysis of 5a(Fig. 3) and the stability of the boat conformation of the hexatomic ring.

|
Download:
|
| Fig. 3. X-ray structure of5a. | |
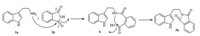
The mechanism of condensation of tryptamine1aand phthalic anhydride2awas proposed in Scheme 2. The most electrophilic carbonyl carbon at the position 1 of phthalic anhydride 2a undergoes nucleophilic attack by tryptamine to afford the intermediate 6,which exhibits amide function as possible nucleophilic groups. Then intermediate 6 undergoes nucleophilic attack once more to give the product3a.
|
Download:
|
| Scheme 2.Proposed mechanism of condensation of tryptamine and phthalic anhydride. | |
In summary,an efficient synthesis of tetrahydro-b-carboline derivatives with strictosamide skeleton has been developed. The reactions are easy to perform,broad in scope,and provide the desired poly-cyclic compounds in good yields. Further efforts to expand and apply these findings are currently underway and will be reported in due course. Acknowledgments
We thank the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1174),the National Natural Science Foundation of China (Nos. 20872118,30070905),the Key Lab Fund of Shaanxi Province of China (Nos. 2010JS097,11JS090, 12JS110) and the Foundation of the Education Department of Shaanxi Province (No. 12JK1010). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.06.012.
| [1] | E. Ernst, M.H. Pittler, Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials, J. Urol. 159 (1998) 433-436. |
| [2] | M. Somei, F. Yamada, Simple indole alkaloids and those with a non-rearranged monoterpenoid unit, Nat. Prod. Rep. 21 (2004) 278-311. |
| [3] | Y.J. Xu, K. Foubert, L. Dhooghe, et al., Chromatographic profiling and identification of two new iridoid-indole alkaloids by UPLC-MS and HPLC-SPE-NMR analysis of an antimalarial extract from Nauclea pobeguinii, Phytochem. Lett. 5 (2012) 316- 319. |
| [4] | (a) E.D. Cox, J.M. Cook, The Pictet-Spengler condensation: a new direction for an old reaction, Chem. Rev. 95 (1995) 1797-1842; (b) M. Rueping, C.M.R. Volla, Brønsted-acid catalyzed condensation-Michael reaction-Pictet-Spengler cyclization—highly stereoselective synthesis of indolo quinolizidines, RSC Adv. 1 (2011) 79-82; (c) J. Stockigt, A.P. Antonchick, F. Wu, H. Waldman, The Pictet-Spengler reaction in nature and in organic chemistry, Angew. Chem. Int. Ed. 50 (2011) 8538-8564; (d) E. Airiau, N. Girard, A. Mann, J. Salvadori, M. Taddei, Four-component reactions toward fused heterocyclic rings, Org. Lett. 11 (2009) 5314-5317;(e) W. Wang, E. Herdtweck, A. Dö mling, Polycyclic indole alkaloid type compounds by MCR, Chem. Commun. 46 (2010) 770-772. |
| [5] | (a) L.S. Santos, C. Theoduloz, R.A. Pilli, J. Rodriguez, Antiproliferative activity of arborescidine alkaloids and derivatives, Eur. J. Med. Chem. 44 (2009) 3810-3815; (b) W.A. da Silva, M.T. Rodrigues Jr., N. Shankaraiah, et al., A novel supramolecular palladium catalyst for the asymmetric reduction of imines in water, Org. Lett. 11 (2009) 3238-3241; (c) M.S. Chern, Y.K. Shih, P.M. Dewang, W.R. Li, Traceless solid-phase synthesis of carbolinones, J. Comb. Chem. 6 (2004) 855-858. |
| [6] | (a) I.T. Raheem, P.S. Thiara, E.A. Peterson, E.N. Jacobsen, Enantioselective Pictet- Spengler-type cyclizations of hydroxylactams: H-bond donor catalysis by anion binding, J. Am. Chem. Soc. 129 (2007) 13404-13405; (b) Atta-ur-Rahman, M. Ghazala, N. Sultana, M. Bashir, Metal ion-catalysed reduction of indolic imides, a facile b-carboline synthesis, Tetrahedron Lett. 21 (1980) 1773-1774. |
| [7] | Y. Zhang, R.P. Hsung, X. Zhang, et al., Brønsted acid-catalyzed highly stereoselective arene-ynamide cyclizations. A novel keteniminium Pictet-Spengler cyclization in total syntheses of ( )-desbromoarborescidines A and C, Org. Lett. 7 (2005) 1047-1050. |
| [8] | (a) C.A. Erdelmeier, A.D. Wright, J. Orjala, et al., New indole alkaloid glycosides from Nauclea orientalis, Planta Med. 57 (1991) 149-152; (b) W. Ma, T. Ling, Y. Zhang, L. Lin, Chemical constituents from Nauclea officinalis, J. Trop. Subtrop. 13 (2005) 167-170. |
| [9] | (a) J.H.A. Paul, A.R. Maxwell, W.F. Reynolds, Novel bis(monoterpenoid) indole alkaloids from Psychotria bahiensis, J. Nat. Prod. 66 (2003) 752-754; (b) F.M. Farias, E.L. Konrath, J.S. Zuanazzi, A.T. Henriques, Strictosamide from Psychotria nuda (ChamEt Schltdl) Wawra (Rubiaceae), Biochem. Syst. Ecol. 36 (2009) 919-920. |
| [10] | Y. Yamazaki, A. Urano, H. Sudo, et al., Metabolite profiling of alkaloids and strictosidine synthase activity in camptothecin producing plants, Phytochemistry 62 (2003) 461-470. |
| [11] | S. Mangalaraj, C.R. Ramanathan, Construction of tetrahydro-β-carboline skeletons via Brønsted acid activation of imide carbonyl group: syntheses of indole alkaloids (±)-harmicine and (±)-10-desbromoarborescidine-A, RSC Adv. 2 (2012) 12665-12669. |
| [12] | Q. Yin, S.G. Wang, S.L. You, Asymmetric synthesis of tetrahydro-β-carbolines via chiral phosphoric acid catalyzed transfer hydrogenation reaction, Org. Lett. 15 (2013) 2688-2691. |
| [13] | J.G.A. Walton, S. Patterson, G. Liu, et al., Synthesis and biological evaluation of functionalised tetrahydro-β-carboline analogues as inhibitors of Toxoplasma gondii invasion, Org. Biomol. Chem. 7 (2009) 3049-3060.. |