It is generally acknowledged that there is an increasing demand for more environmentally acceptable processes,which would feature the elimination of waste at the source and the avoidance of using toxic and/or hazardous substances [1]. Without question,using water as a reaction medium and/or molecular oxygen as an oxidant is consistent with the principles of green chemistry,because water is considered a safe and environmentally benign solvent on account of its massive abundance,non-toxicity and non-inflammability [2]. On the other hand,molecular oxygen is reputed to be an ideal oxidant due to its natural,inexpensive,and eco-friendly characters,with water as the sole byproduct [3].
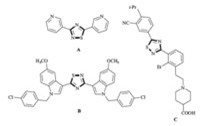
1,2,4-Thiadazoles are recognized as an important class of heterocyclic molecules,which exhibit remarkable biological activities and are broadly found in bioorganic and medicinal chemistry with applications in drug discovery and development [4]. For example,3,5-bis(indolyl)-1,2,4-thiadiazole derivative A (Fig. 1) showed potent cytotoxicity against human cancer line and 3,5-bis(pyridin-3-yl)-1,2,4-thiadiazole B (Fig. 1) is identified as an inhibitor of aromatase for cancer treatment [5, 6]. Thiadiazole compound C (Fig. 1) was developed as an agonists of sphingosine 1-phosphate receptor subtype 1 for the treatment of autoimmune diseases,such as multiple sclerosis and rheumatoid arthritis [7].
|
Download:
|
| Fig. 1. Examples of bioactive 1,2,4-thiadiazoles. | |
In view of the importance of 1,2,4-thiadiazoles,a number of processes have been developed to prepare this class of heterocyclic compound [4, 8]. Among them,the oxidative dimerization of thioamides is the main strategy,which can be carried out by using different oxidizing agents,such as hypervalent iodine [9],alphabromo nitriles [10],and DMSO with the help of an electrophilic reagent [11],and so on [12]. Although able to provide high yields of 1,2,4-thiadiazoles,these reported protocols suffer from drawbacks,such as toxic oxidants and/or hazardous,organic solvents. Herein,we present a green method for the synthesis of 1,2,4-thiadiazole heterocycles through iodine-catalyzed oxidative dimerization of thioamides in water using molecular oxygen as an oxidant (Scheme 1).

|
Download:
|
| Scheme 1.Green process for the synthesis of 1,2,4-thiadiazoles. | |
All reagents were of analytical grade and obtained from commercial suppliers and used without further purification. The 1H NMR and 13C NMR spectra were recorded on a 400 MHz NMR spectrometer,using CDCl3 as the solvent with tetramethylsilane (TMS) as an internal standard at room temperature. Mass spectra were obtained with a SHIMADZU model GCMS-QP5000 spectrometer. High-resolution mass spectra were performed on Finnigan MAT 95 spectrometer. TLC was performed by using commercially prepared 100-400 mesh silica gel plates (GF254) and visualization was effected at 254 nm.
Synthesis of 1,2,4-thiadiazoles: Thioamide (1 mmol),I2 (30 mol%),H2SO4 (50 mol%),hexadecyltrimethylammonium chloride (HTAC) (5 mol%) and water (2 mL) were added to a test tube attached to an oxygen balloon (1 atm). The system was stirred magnetically at r.t. for 12 h. The precipitate was collected by filtration. In the cases of 2a,2b,2d,2g,2h,2k,2l,2r,the pure products were obtained as precipitates and were washed with methanol/water and dried. In other cases,the precipitates were purified by TLC to give pure 1,2,4-thiadiazole products. The characterization data and NMR spectra of the products can be found in Supporting information 3. Results and discussion
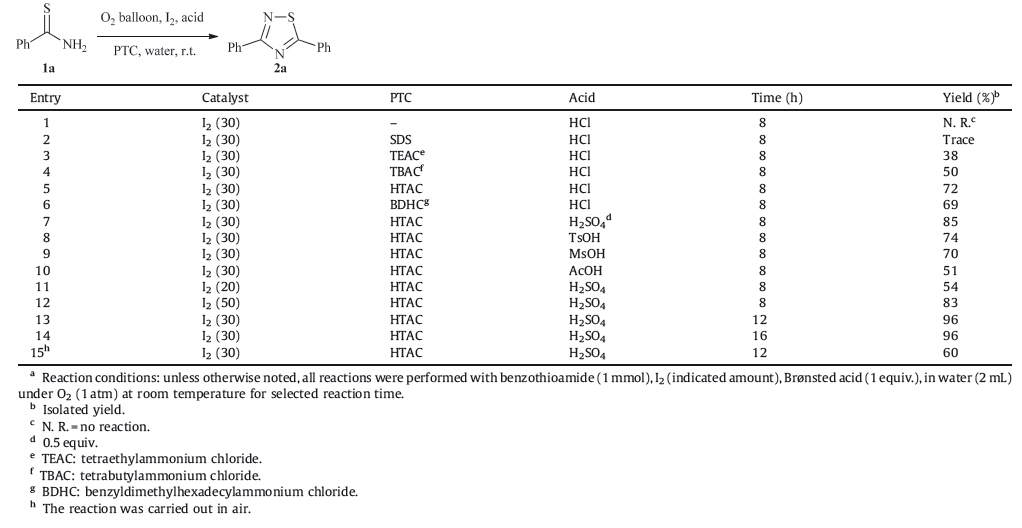
In order to explore the optimum reaction conditions,benzothioamide was selected as a model substrate. The experimental data are collected in Table 1. When benzothioamide reacted in water at room temperature for 12 h,using 20 mol% molecular iodine as the catalyst,in the presence of 1.0 equiv. of aqueous HCl,under 1 atm of pressure of dioxygen,the desired 1,2,4-thiadiazole was not detected by GC (Table 1,entry 1). Usually,the phase transfer catalyst can result in faster organic reactions in water,higher conversions or yields,and fewer byproducts. Based on this consideration,sodium dodecylbenzenesulfonate (SDS) was introduced into this reaction system and it was determined that SDS could improve the transformation slightly (Table 1,entry 2). Our experimental results suggested that quaternary ammonium salts were better phase transfer catalysts and hexadecyltrimethylammonium chloride (HTAC) was found to be the best one (Table 1,entries 3-6). Brønsted acids were then screened,for the reason that the regeneration of the iodine catalyst required protons. As shown in Table 1,sulphuric acid gave the highest yield of the target compound (Table 1,entry 7),while organic protonic acids,such as p-toluenesulfonic acid (TsOH),methylsulfonic acid (MsOH) and acetic acid,were not the preferred proton donors (Table 1,entries 8-10). When the loading of the iodine catalyst was regulated from 30 to 20 mol%,the yield of 2a decreased from 85% to 54% (Table 1,entry 11). However,increasing the amount of the catalyst further was not beneficial (Table 1,entry 12). Further investigations on other influencing factors revealed that 12 h was optimal for the process and a lower yield was realized when the reaction proceeded in air (Table 1,entries 13-15).
| Table 1 Optimization of reaction conditions for the synthesis of 1,2,4-thiadiazoles from thioamides in water using oxygen as an oxidant.a |
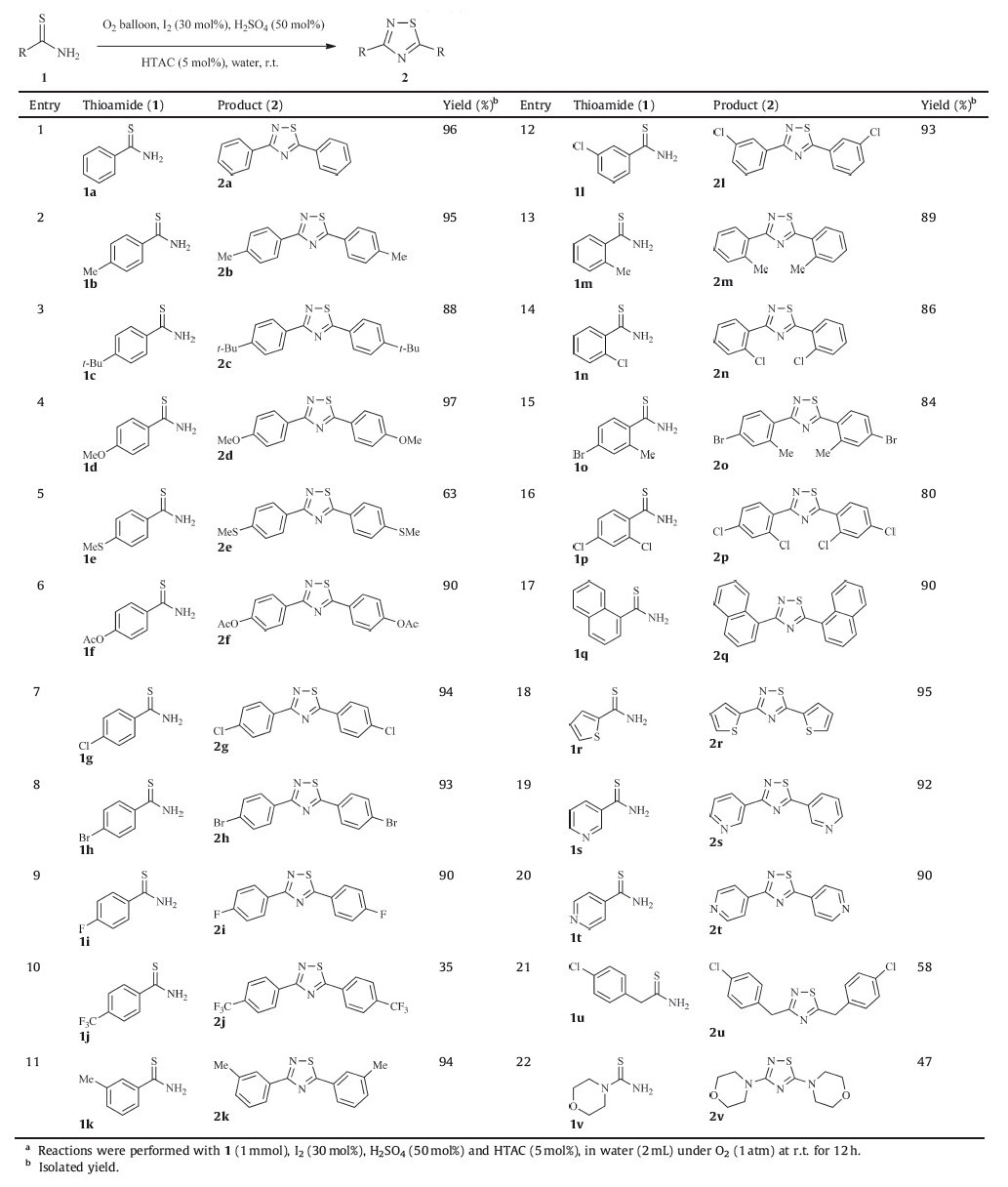
With the optimized reaction conditions in hand,we next evaluated the generality and scope of the methodology for the synthesis of 1,2,4-thiadiazoles from thioamides in water using molecular oxygen as an oxidant. The results are listed in Scheme 2,indicating 4-methylbenzothioamide underwent the transformation almost as efficiently as benzothioamide (2a-b),while 4-tertbutylbenzothioamide gave the corresponding 1,2,4-thiadiazole in a lower yield (2c),probably due to the substituent bulk. As shown in Scheme 2,4-methoxyl,4-methythiol and 4-acetoxyl substituted benzothioamide afforded the target molecules in 97%,63% and 90% yield,respectively (2d-f). Also,4-halogen substituted benzothioamides were all good substrates (2g-i). However,4-(trifluoromethyl) benzothioamide provided the desired heterocyclic compound in an unsatisfactory 35% yield (2j),which indicated that electron-withdrawing substituent on the benzene ring of benzothioamide failed to undergo the dimerization reaction. The 3-substituted benzothioamides undergo the transformation more efficiently than 2-substituted ones (2m-n),but less efficiently than 4-substituted ones (2k-l). In comparison with mono-substituted phenyl thioamides,2,4-disubstituted benzothioamides gave lower yields of the corresponding 1,2,4-thiadiazole products (2o-p). Other hetero-aryl-thioamides were then examined and,as expected,2q,2r,2s,2t were obtained in good to excellent yields when naphthalene-1-carbothioamide,thiophene-2-carbothioamide,pyridine-3-carbothioamide and pyridine-4-carbothioamide were subjected to the optimal reaction conditions. Unexpectedly,alkyl thioamide and thiourea could also undergo this reaction,but provided lower yields (2u-v) (Table 2).
|
Download:
|
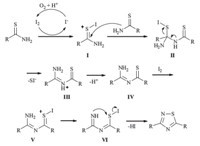
| Scheme 2.Possible mechanism of iodine-catalyzed oxidative dimerization of thioamides. | |
| Table 2 Scope of thioamides for synthesis of 1,2,4-thiadiazoles in water using oxygen as an oxidant.a |
To investigate the applicability of this method for large-scale synthesis,5.0018 g of benzothioamides was allowed to react overnight in a 150 mL round bottom flash charged with 2.7816 g iodine,0.5947 g HTAC and 75 mL 0.25 mol/L H2SO4 connecting to an oxygen balloon,under magnetic stirring at r.t. The precipitate was collected by filtration,washed with methanol/water,and dried. The product 3,5-diphenyl-1,2,4-thiadiazole,in the amount of 4.0493 g,was obtained in 93% yield.
Based on the experimental results mentioned above and the related studies,a probable mechanism of the oxidative dimerization of thioamide is described in Scheme 2. The transformation is initiated by iodination of the sulfur of the thioamide with molecular iodine to form intermediate Ⅰ,which is attacked by another thioamide,as nucleophile,to furnish intermediated Ⅲ. Elimination of HSI will afford intermediate Ⅳ,of which sulfur is then iodination. After tautomerization and elimination of HI,a 1,2,4-thioazole product will be given. The resultant iodide anion throughout the transformation is oxidized by oxygen under the acidic conditions to regenerate the molecular iodine.
In conclusion,we have developed a green procedure for the preparation of 3,5-disubstituted 1,2,4-thiadiazoles through the oxidative dimerizaton of thioamides in water by the catalysis of iodine using molecular oxygen as an oxidant. This method had application to a broad scope of substances,including aryl thioamides,alkyl thioamides and thiaureas. Acknowledgment We thank Guangdong Medical College (No. XK1110) for financial support. Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.019.
| [1] | (a) W.J.W. Watson, How do the fine chemical, pharmaceutical, and related industries approach green chemistry and sustainability? Green Chem. 14 (2012) 251-259; (b) P.J. Dunn, The importance of green chemistry in process research and development, Chem. Soc. Rev. 41 (2012) 1452-1461; (c) P.J. Dunn, Pharmaceutical green chemistry process changes - how long does it take to obtain regulatory approval? Green Chem. 15 (2013) 3099-3104. |
| [2] | (a) M.O. Simona, C.J. Li, Green chemistry oriented organic synthesis in water, Chem. Soc. Rev. 41 (2012) 1415-1427; (b) R.N. Butler, A.G. Coyne, Water: nature's reaction enforcer-comparative effects for organic synthesis "in-water" and "on-water", Chem. Rev. 110 (2010) 6302- 6337; (c) T. Chang, L.Q. He, L. Bian, et al., Brønsted acid-surfactant-combined catalyst for the Mannich reaction in water, RSC Adv. 4 (2014) 727-731. |
| [3] | (a) W.Q. Wu, H.F. Jiang, Palladium-catalyzed oxidation of unsaturated hydrocarbons using molecular oxygen, Acc. Chem. Res. 45 (2012) 1736-1748; (b) Z.Z. Shi, C. Zhang, C.H. Tang, N. Jiao, Recent advances in transition-metal catalyzed reactions using molecular oxygen as the oxidant, Chem. Soc. Rev. 41 (2012) 3381-3430; (c) G.Y. Liu, R.R. Tang, Z.Wang, Metal-free allylic oxidation with molecular oxygen catalyzed by g-C3N4 and N-hydroxyphthalimide, Catal. Lett. 144 (2014) 717-722. |
| [4] | A. Castro, T. Castañ o, A. Encinas, W. Porcal, C. Gil, Advances in the synthesis and recent therapeutic applications of 1,2,4-thiadiazole heterocycles, Bioorg. Med. Chem. 14 (2006) 1644-1652. |
| [5] | A.S. Mayhoub, L. Marler, T.P. Kondratyuk, R. Gupta, K. Shah, Optimizing thiadiazole analogues of resveratrol versus three chemopreventive targets, Bioorg. Med. Chem. 20 (2012) 510-520. |
| [6] | D. Kumar, N.M. Kumar, K.H. Chang, et al., Synthesis and in-vitro anticancer activity of 3,5-bis(indolyl)-1,2,4-thiadiazoles, Bioorg. Med. Chem. Lett. 21 (2011) 5897-5900. |
| [7] | F. Ren, G.H. Deng, H.L. Wang, et al., Discovery of novel 1,2,4-thiadiazole derivatives as potent, orally active agonists of sphingosine 1-phosphate receptor subtype 1 (S1P1), J. Med. Chem. 55 (2012) 4286-4296. |
| [8] | (a) E.A.F. Fordyce, A.J. Morrison, R.D. Sharp, et al., Microwave-induced generation and reactions of nitrile sulfides: an improved method for the synthesis of isothiazoles and 1,2,4-thiadiazoles, Tetrahedron 66 (2010) 7192-7200; (b) H.A. Beeley, S. Degorce, C.S. Harris, et al., One-pot synthesis of bis(amino)- 1,2,4-thiadiazoles via direct SNAr, Tetrahedron Lett. 54 (2013) 788-791; (c) J. Noei, A.R. Khosropour, A novel process for the synthesis of 3,5-diaryl-1,2,4- thiadiazoles from aryl nitriles, Tetrahedron Lett. 54 (2013) 9-11; (d) Y.L. Xu, J.X. Chen, W.X. Gao, et al., Solvent-free synthesis of 3,5-di(hetero)aryl- 1,2,4-thiadiazoles by grinding of thioamides under oxidative conditions, J. Chem. Res. 34 (2010) 151-153. |
| [9] | (a) A.A. Shah, Z.A. Khan, N. Choudhary, et al., Iodoxolone-based hypervalent iodine reagents, Org. Lett. 11 (2009) 3578-3581; (b) P.C. Patil, D.S. Bhalerao, P.S. Dangate, et al., IBX/TEAB-mediated oxidative dimerization of thioamides: synthesis of 3,5-disubstituted 1,2,4-thiadiazoles, Tetrahedron Lett. 50 (2009) 5820-5822. |
| [10] | (a) A.S. Mayhoub, E. Kiselev, M. Cushman, An unexpected synthesis of 3,5-diaryl- 1,2,4-thiadiazoles from thiobenzamides and methyl bromocyanoacetate, Tetrahedron Lett. 52 (2011) 4941-4943; (b) H.Z. Boeini, M. Mobin, An unexpected result of the reaction of benzothioamide derivatives with 2-aryl-2-bromoacetonitriles, Helv. Chim. Acta 94 (2011) 2039-2044. |
| [11] | (a) A.R. Khosropour, J. Noei, TCT-DMSO/[bmim]BF4: a novel promoter system for the synthesis of 3,5-diaryl-1,2,4-thiadiazoles at ambient temperature, J. Heterocycl. Chem. 48 (2010) 226-229; (b) Y. Takikawa, K. Shimada, K. Sato, et al., Convenient preparations of 3,5- disubstituted 1,2,4-thiadiazoles by oxidative dimerization of thioamides, Bull. Chem. Soc. Jpn. 58 (1985) 995-999. |
| [12] | (a) T. Fukumoto, T. Matsuki, N.X. Hu, Benzenetellurinic mixed anhydrides as mild oxidizing agents, Chem. Lett. 19 (1990) 2269-2272; (b) M.T.M. El-Wassimy, K.A. Jorgensen, S.O. Lawesson, The reaction of t-butyl hypochlorite with thiocarbonyl compound - a convenient method for the transformation, Tetrahedron 39 (1983) 1729-1734. |