One-pot,multicomponent strategies are used to improve the efficiency of chemical reactions,whereby multiple carbon-carbon and carbon-heteroatom and stereocenters are formed in a single operation without the need to isolate intermediates and represents an efficient approach to the synthesis of complex molecular structures based on simple organic building blocks [1, 2, 3, 4, 5].
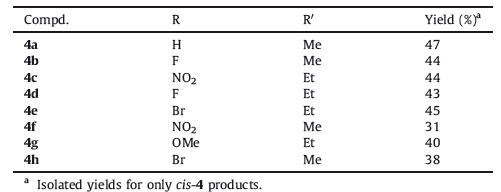
A 1,4-dipolar cycloaddition provides an efficient and convenient route for the synthesis of six-membered heterocyclic compounds. Extensive work has been done on the reactivities of 1,4-dipoles derived from dimethyl acetylenedicarboxylate (DMAD) and nucleophiles,such as nitrogen heterocycles [6, 7, 8, 9, 10]. These studies have led to a number of interesting carbon- heteroatom bond-forming reactions and heterocyclic constructions [11, 12, 13]. The reactivity of nitrogen-containing heterocycles,such as pyridine and isoquinoline as nucleophiles,with activated alkynes,such as dimethyl acetylenedicarboxylate (DMAD),in the presence of carbon or hydrogen-electrophiles is well documented [6]. The reaction generally involves the initial addition of pyridine or isoquinoline to activated alkynes to form zwitterionic intermediates which can be trapped by various electrophiles. Trapping of the pyridine-DMAD zwitterion and the isoquinoline-DMAD zwitterion with aldehydes has been reported [6, 7, 8, 9, 10]. Whereas the pyridine-DMAD zwitterion induced novel molecular rearrangements [6, 7],the isoquinoline-DMAD zwitterion engaged exclusively in three component reactions [8, 9, 10]. The addition of quinoline-DMAD zwitterion to aldehydes and 1,2-diones has also been reported to afford three-component addition products [14]. As part of the study on the development of the reaction of nucleophiles,nitrogen-containing heterocycles [14] and our previous studies on the development and synthesis of phenanthridine derivatives [15, 16],we report here the results of our studies involving the reactions of phenanthridine,activated acetylenes,and aromatic aldehydes for synthesis of new phenanthridine derivatives in dry dichloromethane for 24 h (Scheme 1).
|
Download:
|
| Scheme 1.The synthesis of phenanthridine derivatives. | |
The chemicals used in this work were purchased from Merck and Aldrich chemical companies. Melting points were determined using a Mettler FP5 apparatus and are uncorrected. IR spectra were determined using KBr pellets on a Shimadzu recording spectrophotometer,Model 435. The 1H NMR and 13C NMR (400 and 100 MHz) spectra were recorded on a Bruker 400 spectrometer in DMSO with TMS as internal standard. Elemental analyses were performed using a Carlo Erba EA 1108 instrument. 2.1. Representative experimental procedure
To a mixture of benzaldehyde (53.0 mg,0.50 mmol) and phenanthridine (68.45 mg,0.53 mmol) in dry dichloromethane (5 mL) was added dropwise dimethyl acetylenedicarboxylate (72.50 mg,0.50 mmol) in dry dichloromethane (2 mL) at room temperature. The reaction mixture was further stirred for 24 h. Removal of the solvent followed by purification of the reaction mixture by column chromatography (silica gel,100-200 mesh; nhexane/ ethyl acetate (90:10,v/v)) afforded 4a as a yellow solid which could be recrystallized from ethyl acetate/hexane (1:1,v/v). 2.2. Physical and spectroscopic data for compound 4a-4h
Compound 4a: Yield 47% (200 mg); Mp 184-186℃. IR (KBr,cm-1): υmax 3037,1740,1712,1597,1511,1429,1264,1219,1010,757. 1H NMR (400 MHz,DMSO-d6): δ 8.03 (d,2H,J = 7.6 Hz),7.51 (dd,2H,J = 7.6 Hz),7.38 (d,2H,J = 8.0 Hz),7.32 (d,1H,J = 8.0 Hz),7.25 (dd,2H,J = 7.6 Hz),7.13 (dd,1H,J = 7.6 Hz),7.05 (dd,1H,J = 7.6 Hz),6.94 (dd,1H,J = 7.6 Hz),6.81 (t,1H,J = 7.6 Hz),6.02 (s,1H),5.71 (s,1H),3.77 (s,3H),3.52 (s,3H). 13C NMR (100 MHz,DMSO-d6): δ 166.19,162.47,145.71,142.23,140.54,135.29,131.85,130.12,129.85,129.04,128.38,127.73,125.83,125.52,123.82,
122.09,121.75,119.72,113.83,81.72,79.69,74.15,60.81,58.50. Anal. Calcd. for C26H21NO5 (427.14): C,73.06; H,4.95; N,3.28. Found: C,73.19; H,4.96; N,3.26.
Compound 4b: Yield 44% (195 mg); Mp 206-208 ℃. IR (KBr,cm-1): υmax 3042,1736,1709,1599,1504,1438,1258,1221,1011,752. 1H NMR (400 MHz,DMSO-d6): δ 8.11 (d,2H,J = 7.6 Hz),7.55 (dd,2H,J = 7.6 Hz),7.40 (d,2H,J = 8.0 Hz),7.28 (d,1H,J = 8.0 Hz),7.20 (dd,2H,J = 7.6 Hz),7.10 (dd,1H,J = 7.6 Hz),7.07 (d,1H,J = 7.6 Hz),6.90 (d,1H,J = 7.6 Hz),6.06 (s,1H),5.94 (s,1H),3.80 (s,3H),3.61 (s,3H). 13C NMR (100 MHz,DMSO-d6): δ 165.12,164.97,146.80,145.88,140.17,137.44,130.57,130.35,129.97,129.91,128.64,128.07,127.12,124.04,122.93,
122.19,121.68,120.46,115.47,82.10,78.25,77.69,61.94,60.73. Anal. Calcd. for C26H20FNO5 (445.13): C,70.11; H,4.53; N,3.14. Found: C,70.23; H,4.50; N,3.16.
Compound 4c: Yield 44% (220 mg); Mp 206-208 ℃. IR (KBr,cm-1): υmax 3057,1730,1710,1600,1513,1437,1258,1224,1010,754. 1H NMR (400 MHz,DMSO-d6): δ 8.13 (d,4H,J = 7.0 Hz),7.56 (d,2H,J = 6.0 Hz),7.44 (d,4H,J = 7.6 Hz),7.20 (dd,1H,J = 7.6 Hz),6.97 (d,1H,J = 7.6 Hz),6.12 (s,1H),6.10 (s,1H),4.33 (q,1H,J = 7.2 Hz),4.25 (q,1H,J = 7.2 Hz),4.12 (q,2H,J = 7.2 Hz),1.22 (t,3H,J = 7.2 Hz),1.11 (t,3H,J = 7.2 Hz). 13C NMR (100 MHz,DMSO-d6): δ 164.29,163.75,147.80,146.78,141.17,137.34,130.59,130.25,129.66,129.61,128.59,128.25,128.04,
125.04,123.93,123.19,122.68,121.46,116.47,83.09,79.65,77.69,62.74,61.66,14.08,14.01. Anal. Calcd. for C28H24N2O7 (500.16): C,67.19; H,4.83; N,5.60. Found: C,67.15; H,4.79; N,5.61.
Compound 4d: Yield 43% (203 mg); Mp 191-193 ℃. IR (KBr,cm-1): υmax 3008,1737,1712,1616,1526,1439,1350,1265,1225,1011,754. 1H NMR (400 MHz,DMSO-d6): δ 8.06 (d,1H,J = 6.8 Hz),8.04 (d,1H,J = 7.6 Hz),7.56 (d,2H,J = 8.0 Hz),7.51 (dd,1H,J = 7.6 Hz),7.37 (d,2H,J = 8.0 Hz),7.28 (dd,2H,J = 7.6 Hz),7.17 (dd,1H,J = 7.6 Hz),7.07 (d,1H,J = 7.6 Hz),6.87 (d,1H,J = 7.6 Hz),5.74 (s,1H),5.69 (s,1H),4.26 (q,2H,J = 7.0 Hz),4.01 (q,2H,J = 7.0 Hz),1.22 (t,3H,J = 7.0 Hz),1.00 (t,3H,J= 7.0 Hz). 13C NMR (100 MHz,DMSO-d6): δ 163.28,161.15,148.08,147.90,143.69,137.01,131.17,130.09,129.46,128.93,128.51,128.15,127.44,126.14,123.73,123.09,122.08,
120.78,114.57,82.41,79.05,78.59,61.74,59.06,15.12,15.07. Anal. Calcd. for C28H24FNO5 (473.15): C,71.03; H,5.11; N,2.96. Found: C,70.92; H,5.09; N,2.97.
Compound 4e: Yield 45% (239 mg); Mp 195-197 ℃. IR (KBr,cm-1): υmax 3035,1731,1707,1598,1501,1445,1269,1220,1011,753. 1H NMR (400 MHz,DMSO-d6): δ 8.13 (d,1H,J= 6.8 Hz),8.11 (d,1H,J= 7.6 Hz),7.54 (d,2H,J= 7.6 Hz),7.45 (d,2H,J= 8.0 Hz),7.41 (dd,2H,J= 7.6 Hz),7.17 (dd,2H,J= 7.6 Hz),7.12 (d,2H,J= 8.0,Hz),6.95 (d,1H,J= 7.6 Hz),6.06 (s,1H),5.92 (s,1H),4.25 (q,2H,J= 8.0 Hz),4.04 (q,2H,J= 8.0 Hz),1.22 (t,3H,J= 8.0 Hz),1.09 (t,3H,J= 8.0 Hz). 13C NMR (100 MHz,DMSO-d6): δ 165.07,162.87,150.32,147.94,144.61,137.91,133.24,130.76,129.83,128.55,128.03,127.83,127.06,125.61,123.03,122.84,122.06,
121.98,115.81,81.71,77.37,74.41,62.16,60.85,16.29,15.30. Anal. Calcd. for C28H24BrNO5 (533.08): C,62.93; H,4.53; N,2.62. Found: C,62.85; H,4.52; N,2.60.
Compound 4f: Yield 31% (146 mg); Mp 118-120℃. IR (KBr,cm-1): υmax 3073,1725,1707,1607,1502,1446,1259,1224,1011,754. 1H NMR (400 MHz,DMSO-d6): δ 8.14 (d,2H,J = 7.0 Hz),8.12 (d,2H,J = 7.0 Hz),7.55 (d,2H,J = 6.0 Hz),7.45 (d,4H,J = 7.6 Hz),7.20 (dd,1H,J = 7.6 Hz),6.94 (d,1H,J = 7.6 Hz),6.11 (s,2H),3.82 (s,3H),3.63 (s,3H). 13C NMR (100 MHz,DMSO-d6): δ 164.75,164.33,147.82,146.68,141.34,137.19,130.60,130.40,129.66,129.62,129.51,128.60,127.98,125.07,123.96,123.26,122.68,
121.48,116.31,83.14,77.64,60.22,53.73,52.79. Anal. Calcd. for C26H20N2O7 (472.13): C,66.10; H,4.27; N,5.93. Found: C,66.24; H,4.25; N,5.94.
Compound 4g: Yield 40% (194 mg); Mp 180-184 ℃. IR (KBr,cm-1): υmax 3028,1737,1713,1593,1510,1436,1259,1217,1009,751. 1H NMR (400 MHz,DMSO-d6): δ 8.05 (d,1H,J = 6.8 Hz),8.03 (d,1H,J = 6.8 Hz),7.51 (dd,1H,J = 7.6 Hz),7.42 (d,2H,J = 8.0 Hz),7.34 (d,1H,J = 6.8 Hz),7.32 (d,1H,J = 7.6 Hz),7.17 (dd,1H,J = 7.6 Hz),7.06 (d,1H,J = 7.6 Hz),6.99 (d,2H,J = 8.0 Hz),6.88 (d,1H,J = 7.6 Hz),5.99 (s,1H),5.62 (s,1H),4.26 (q,2H,J = 7.2 Hz),4.02 (q,2H,J = 7.2 Hz),3.77 (s,3H),1.22 (t,3H,J = 7.2),1.02 (t,3H,J = 7.2 Hz). 13C NMR (100 MHz,DMSO-d6): δ 165.26,163.57,159.94,139.00,136.86,131.42,130.27,130.13,130.08,129.77,128.94,128.60,127.75,125.03,123.68,123.00,122.91,
122.13,117.71,114.54,78.66,74.51,62.64,61.20,55.59,14.14,13.97. Anal. Calcd. for C29H27NO6 (485.18): C,71.74; H,5.61; N,2.88. Found: C,71.72; H,5.65; N,2.89.
Compound 4h: Yield 38% (191 mg); Mp 205-207 ℃. IR (KBr,cm-1): υmax 3039,1737,1711,1607,1437,1256,1220,1010,754. 1H NMR (400 MHz,DMSO-d6): δ 8.03 (d,2H,J = 7.6 Hz),7.78 (dd,1H,J = 7.6 Hz),7.66 (d,2H,J = 8.0 Hz),7.46 (d,2H,J = 8.0 Hz),7.35 (dd,2H,J = 7.6 Hz),7.19 (dd,1H,J = 7.6 Hz),7.12 (d,1H,J = 7.6 Hz),6.87 (d,1H,J = 7.6 Hz),5.79 (s,1H),5.63 (s,1H),3.81 (s,3H),3.57 (s,3H). 13C NMR (100 MHz,DMSO-d6): δ 163.31,162.51,147.93,145.38,142.19,135.04,131.53,130.62,129.73,129.27,128.51,128.14,127.11,125.72,123.41,123.11,122.53,
121.07,117.55,85.48,78.61,76.52,61.64,59.74. Anal. Calcd. for C26H20BrNO5 (505.05): C,61.67; H,3.98; N,2.77. Found: C,61.91; H,3.98; N,2.75.
Compound 6: Yield 35% (176 mg); Mp 273-274 ℃. IR (KBr,cm-1): υmax 3012,1762,1719,1704,1610,1505,1438,1274,1203,1007,750. 1H NMR (400 MHz,DMSO-d6): δ 8.34 (d,1H,J = 8.0 Hz),8.31 (d,1H,J = 8.0 Hz),8.17 (dd,2H,J = 8.0 Hz),8.13 (d,1H,J = 8.0 Hz),7.96 (d,1H,J = 8.0 Hz),7.84 (dd,1H,J = 7.6 Hz),7.83 (d,1H,J = 7.6 Hz),7.53 (d,1H,J = 7.6 Hz),7.45 (dd,1H,J = 7.6 Hz),7.36 (dd,1H,J = 7.6 Hz),7.23 (d,1H,J = 7.6 Hz),7.21 (d,1H,J = 7.6 Hz),6.91 (d,1H,J = 7.6 Hz),6.50 (s,1H),3.85 (s,3H),3.26 (s,3H). 13C NMR (100 MHz,DMSO-d6): d 179.22,164.17,161.42,152.19,148.21,146.03,141.61,139.22,136.48,131.94,131.17,129.61,129.27,128.51,128.14,127.11,126.27,
125.38,124.83,123.52,123.10,122.72,122.53,121.84,120.45,118.15,83.79,78.14,77.08,60.71,58.26. Anal. Calcd. for C31H21NO6 (503.14): C,73.95; H,4.20; N,2.78. Found: C,74.07; H,4.22; N,2.80.Compound 6: Yield 35% (176 mg); Mp 273-274 ℃. IR (KBr,cm-1): υmax 3012,1762,1719,1704,1610,1505,1438,1274,1203,1007,750. 1H NMR (400 MHz,DMSO-d6): δ 8.34 (d,1H,J = 8.0 Hz),8.31 (d,1H,J = 8.0 Hz),8.17 (dd,2H,J = 8.0 Hz),8.13 (d,1H,J = 8.0 Hz),7.96 (d,1H,J = 8.0 Hz),7.84 (dd,1H,J = 7.6 Hz),7.83 (d,1H,J = 7.6 Hz),7.53 (d,1H,J = 7.6 Hz),7.45 (dd,1H,J = 7.6 Hz),7.36 (dd,1H,J = 7.6 Hz),7.23 (d,1H,J = 7.6 Hz),7.21 (d,1H,J = 7.6 Hz),6.91 (d,1H,J = 7.6 Hz),6.50 (s,1H),3.85 (s,3H),3.26 (s,3H). 13C NMR (100 MHz,DMSO-d6): d 179.22,164.17,161.42,152.19,148.21,146.03,141.61,139.22,136.48,131.94,131.17,129.61,129.27,128.51,128.14,127.11,126.27,
125.38,124.83,123.52,123.10,122.72,122.53,121.84,120.45,118.15,83.79,78.14,77.08,60.71,58.26. Anal. Calcd. for C31H21NO6 (503.14): C,73.95; H,4.20; N,2.78. Found: C,74.07; H,4.22; N,2.80.
3. Results and discussion
The structure of product 4a was deduced by analytical and spectroscopic methods. In the 1HNMR spectrum,signals due to the methoxy carbonyl protons were observed as sharp singlets at δ 3.52 and 3.77. The twomethine protonswere discernible as two singlet signals at δ 5.71 and 6.02. The aromatic protons resonated in the region δ 6.81-8.03. The 13C NMR spectrumof compound 4a exhibited twenty-four distinct signals in agreement with the proposed structure. In the IR spectrum,the ester carbonyl absorptions were observed at 1740 and 1712 cm-1.
Analogous reactions were observed with other aromatic aldehydes,and the results are presented in Table 1. It was observed that the reaction was compatible with different aldehydes and afforded the phenanthridine derivatives in moderate yields.
| Table 1 Reaction of phenantridine,dialkyl acetylenedicarboxylates and aromatic aldehydes(Scheme 1). |
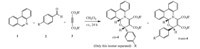
We also investigated the reaction between phenantridine and DMAD in the presence of phenantroquinone 5. After refluxing the reaction mixture in toluene for 24 h and column chromatography,compound 6 was obtained in 35% yield (Scheme 2).
|
Download:
|
| Scheme 2.TThe reaction between phenantridine,DMAD and phenantroquinone. | |
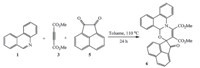
A mechanistic rationalization for the reaction is given in Scheme 3. Nucleophilic attack of 1 on the electron-deficient triple bond 3 could lead to the formation of intermediate A,which possessed a resonance structure B as a 1,4-dipolar synthon. When the aromatic aldehyde 2 existed in the mixture of 1 and 3,a subsequent [4+2] cycloaddition occurred. In this way,the corresponding phenanthridine derivatives 4 were prepared (Scheme 3). During the process of the cycloaddition,there are two possible transition states C and D,which might lead to the formation of two possible products,trans-4 and cis-4 accordingly. The D transition state is more stable than the C transition state. Thus,cis-4 was obtained as the major component in comparison to the trans-4 isomer. Of course,we could only separate the cis-4 product from the mixture of reaction products by column chromatography.
|
Download:
|
| Scheme 3.Proposed mechanistic pathway for synthesis of phenanthridine derivatives. | |
In summary,we report herein the synthesis of new phenanthridine derivatives by a one-pot,three-component reaction between phenantridine,dialkyl acetylenedicarboxylates and aromatic aldehydes via 1,4-dipolar cycloaddition under neutral conditions. This is the first MCR involving a zwitterion generated from phenanthridine and dialkyl acetylenedicarboxylates.
| [1] | J. Zhu, H. Bienayme, Multicomponent Reactions, Wiley-VCH, Weinheim, 2005. |
| [2] | A. Dö mling, Recent developments in isocyanide based multicomponent reactions in applied chemistry, Chem. Rev. 106 (2006) 17-89. |
| [3] | M.A. Mironov, Design of multi-component reactions: from libraries of compounds to libraries of reactions, QSAR Comb. Sci. 25 (2006) 423-431. |
| [4] | A. Padwa, S.K. Bur, The domino way to heterocycles, Tetrahedron 63 (2007) 5341- 5378. |
| [5] | D. Marko, M.D. Mihovilovic, P. Stanetty, Metal-assisted multicomponent reactions involving carbon monoxide-towards heterocycle synthesis, Angew. Chem. Int. Ed. 46 (2007) 3612-3615. |
| [6] | V. Nair, A.R. Sreekanth, A.U. Vinod, Novel pyridine-catalyzed reaction of dimethyl acetylenedicarboxylate with aldehydes: formal [2+2] cycloaddition leading to 2- oxo-3-benzylidinesuccinates, Org. Lett. 3 (2001) 3495-3497. |
| [7] | V. Nair, A.R. Sreekanth, A.U. Vinod, Novel pyridine-catalyzed reaction of dimethyl acetylenedicarboxylate with aldehydes: formal [2+2] cycloaddition leading to 2- oxo-3-benzylidenesuccinates, Org. Lett. 4 (2002) 2807. |
| [8] | V. Nair, A.R. Sreekanth, N. Abhilash, M.M. Bhadbhade, R.C. Gonnade, A novel threecomponent reaction for the diastereoselective synthesis of 2H-pyrimido[2,1- a]isoquinolines via 1,4-dipolar cycloaddition, Org. Lett. 4 (2002) 3575-3577. |
| [9] | V. Nair, A.R. Sreekanth, A.T. Biju, N.P. Rath, The reaction of isoquinoline and dimethyl acetylenedicarboxylate with 1,2- and 1,4-benzoquinones: a novel synthesis of spiro[1,3]oxazino[2,3-a]isoquinolines, Tetrahedron Lett. 44 (2003) 729-732. |
| [10] | V. Nair, B.R. Devi, R.L. Varma, The Huisgen 1,4-dipolar cycloaddition involving isoquinoline, dimethyl butynedioate and activated styrenes: a facile synthesis of tetrahydrobenzoquinolizine derivatives, Tetrahedron Lett. 46 (2005) 5333-5335. |
| [11] | V. Nair, C. Rajesh, A.U. Vinod, Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes, Acc. Chem. Res. 36 (2003) 899-907. |
| [12] | V. Nair, R.S. Menon, A. Sreekanth, N. Abhilash, A.T. Biju, Engaging zwitterions in carbon-carbon and carbon-nitrogen bond-forming reactions: a promising synthetic strategy, Acc. Chem. Res. 39 (2006) 520-530. |
| [13] | M. Lei, Z.J. Zhan, W. Tian, P. Lu, One-pot, three-component synthesis of highly functionalized 1,3-oxazine derivatives from a,b-unsaturated imine, alkyne, and aldehyde, Tetrahedron 68 (2012) 3361-3367. |
| [14] | V. Nair, S. Devipriya, E. Suresh, Efficient synthesis of [1,3]oxazino[2,3-a]quinoline derivatives by a novel 1,4-dipolar cycloaddition involving a quinoline-DMAD zwitterion and carbonyl compounds, Tetrahedron Lett. 48 (2007) 3667-3670. |
| [15] | H. Mehrabi, M.A. Hatami-Pour, Synthesis of functionalized pyrido[1,2-f]phenanthridines from phenanthridine, activated acetylenes, and arylidenemalononitriles, Synth. Commun. 43 (2013) 611-618. |
| [16] | H. Mehrabi, J. Pishahang, Synthesis of functionalized pyrido[1,2-f]phenanthridines from phenanthridine, activated acetylenes, and arylidenemalononitriles, Synth. Commun. 44 (2014) 76-81. |