Ethylene glycol (EG) is an important chemical widely used in the production of polyesters [1] and antifreezers,and it is mainly producedviahydration of ethylene oxide [2]. With the shrinking of the crude oil resources,synthesis of EG from non-oil sources like dimethyl oxalate (DMO) attracts more and more attentions both from academic and industrial communities. DMO has been successfully produced from syngas by coupling of CO with nitrite esters over Pd/a-A2O3[3, 4],and then EG can be obtained by the hydrogenation of DMO. Industrial catalysts for the hydrogenation process are Cr/Cu-based catalysts [5],which produce a high yield of EG and possess a satisfactory lifespan. But the toxicity of Cr is the biggest disadvantage that limits its application. Thus,it necessitates the research on the alternative catalysts. Two approaches, namely changing the active metals (like Ru [6, 7],Ag [8]) and improving the copper-based catalysts either by addition of non-Cr elements (like B [9, 10],Ni [11],Ag [12]) or by modifying the preparation procedures (deposition-precipitation (DP) method [13],ion-exchange (IE) method [14, 15],ammonia evaporation (AE) method [1, 16, 17],ammonia evaporation hydrothermal (AEH) method [18],urea hydrolysis (UH) method [19],sol-gel (SG) method [16, 20],one-pot (OP) method [21, 22],etc.),are being intensively studied.
The hydrogenation of DMO to EG is a process with two sequential steps,the first of which is the formation of the intermediate methylene glycollate (MG) and the second one is MG hydrogenation to EG. Side reactions include the intermolecular dehydration of EG over strong acid sites leading to ethanol and the Guerbet reaction [23, 24] between primary aliphatic alcohols (methanol,ethanol and EG) forming 1,2-propanediol (PDO) and 1,2-butanediol (BDO) on basic sites. Thus,neutral or weak acid/ alkaline carriers are more appropriate supports in this reaction [2]. For this reason,SiO2is herein chosen as the support.
Deposition-precipitation method using ammonia as a precipitant can generate well-dispersed and relatively fine CuO particles compared with the conventional methods [16, 17, 18, 19]. So it has always been highly preferable. The amount of ammonia in the DP method is an important parameter because it has a direct influence on the surface area and porosity of the catalysts prepared [13]. However, very little research has been done on the influence of the amount of ammonia on the Cu0/Cu+ ratio,which affects the catalytic performance directly [18, 25]. In this paper,a series of catalysts with different Cu0/Cu+ ratios were prepared viathe DP method using ammonia as a precipitant and applied to the hydrogenation of DMO to EG for activity test. 2. Experimental
Catalyst preparation: The Cu/SiO2catalysts were prepared by the deposition-precipitation method using concentrated ammonia (28 wt%) solution as a precipitant. Typically,the process involves the following three steps. Firstly,the desired amount,xmL of concentrated ammonia solution was added dropwise into a 100 mL of 0.21 mol L-1Cu(NO3)2solution with vigorous stirring and the corresponding pH value of the mixture was measured using a calibrated pH meter (pH-2601),followed by the addition of ultrafine silica (VK-SP15,particle diameter is 15±5 nm,Xuan Cheng Jing Rui New Material Co.,Ltd.),after that the mixture was stirred for 8.5 h and stood still overnight. The precipitate was then washed and filtrated with 15 mL of deionized water twice. Dryness took place at 393 K in air for 7 h along with the calcination procedure under conditions of 723 K in static air for 4 h. The sample usingxmL of concentrated ammonia solution was donated as Wx. Catalysts were then crushed and sieved to collect the 20-40 mesh part.
Catalyst characterization: Textual properties of the catalysts were measured by a N2adsorption method with a NOVA 1000e surface area and pore size analyzer. Mesoporous surface area was calculated from the desorption isotherms using the Brunauer- Emmet-Teller (BET) method and the pore size distribution was determined using the Barrett-Joyner-Halenda (BJH) method according to the desorption branches of the adsorption isotherms. The bulk composition of the samples was determinedviaX-ray fluorescence (XRF). The temperature programmed reduction (TPR) was carried out with a quartz tube (the inner diameter is 8 mm) with a TCD as the detector. A sample of 0.1 g 20-40 mesh catalyst was loaded into the tube and heated from 303 K to 1073 K with a ramping rate of 10 K/min,using a 5%/95% (v/v) H2-Ar (flowing rate is 30 mL min-1 ) as the reducing gas. X-ray powder diffraction (XRD) was obtained on an X’Pert PRO diffractometer using Cu Ka radiation (l= 0.15406 nm) at 45 kV and 40 mA. The scanning range is from 2u=10°-80°with a scanning rate of 28 min-1 . The diffraction peaks obtained were referred to the JCPDS cards. Fourier Transform infrared (FTIR) spectra of the calcined catalysts were recorded at room temperature using a Nicolet 6700 spectrometer.
Activity test and product analysis: Activity test is carried out in a continuous flow unit (WFD 3030,Tianjin Xianquan Industry and Trade Development Co.,Ltd.),equipped with a stainless steel tubular reactor (inner diameter is 8 mm). About 1.0 g of each catalyst was loaded into the tube with both sides packed with an appropriate amount of quartz powders to ensure the plug flow profile of the feed. A thermocouple was inserted into the catalyst bed for the control of the heating. Then the catalyst was activated in pure hydrogen atmosphere (flow rate of 30 mL min-1 ,pressure of 1 atm) at 523 K for 4 h with a ramping rate of 10 K min-1 , followed by natural cooling to the reaction temperature of 473 K. A 15 wt% DMO in methanol solution and pure H2were fed into the reactor with a H2/DMO molar ratio of 60 under a system pressure of 2.0 MPa. The weight hourly space velocity (WHSV) of DMO was 0.65 h -1 . The reaction products were condensed and analyzed by gas chromatography (Agilent 7890A) equipped with an En-20 capillary column (30 m×0.32 mm×0.25mm) and a flame ionization detector (FID). By-products include ethanol,MG,PDO and BDO. Analysis of each product was carried on by the inner standard curve method withn-butanol as the inner standard. 3. Results and discussion 3.1. Characterization of the catalysts
Texture properties were determined by the N2 adsorption method. Table 1 lists the physical properties of the catalysts and the support. The surface area decreases as the amount of ammonia used increases. Both the pore volume and average pore size of all catalysts are larger than those of silica. Initial pH value increases with the increasing of ammonia,while the copper loading descends. These trends will be discussed in detail in the following section. N2 adsorption-desorption isotherms (see Fig. S1(A) in Supporting information) of all calcined samples exhibit Langmuir type IV isotherms with an H1-type hysteresis loop,indicating a typical mesoporous material packed with size-homogeneous spherical particles. Pore size distribution curves (Fig. S1(B) in Supporting information) further confirm the mesoporosity of the catalysts. Compared to the support with an average pore size of 11 nm,it can be seen that the catalysts have two kinds of pores with the sizes of 3 nm and 32 nm,respectively. The smaller pores may be resulted from the filling of copper oxide particles,while the larger ones may be created during the preparation process.
| Table 1 Physical properties of the catalysts. |
|
Download:
|
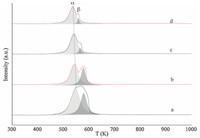
| Fig. 1. The TPR profiles of the calcined catalysts ((a) W2.5; (b) W3; (c) W6; (d) W12). | |
In order to elucidate the effect of ammonia amount on the formation of precursor species,uncalcined samples were characterized by XRD (Fig. S2 in Supporting information). As shown in Fig. S2a and b,two phases,namely chrysocolla and copper nitrate hydroxide (Cu2(OH)3NO3,referred to JCPDS 15-0014) co-exist in both W2.5 and W3,while in samples W6 and W12,shown in Fig. S2c and d,copper hydroxide (referred to JCPDS 03-0315) phase instead of copper nitrate hydroxide accompanies chrysocolla phase. Chrysocolla phase was identified among all samples. The intensity of chrysocolla descends from W2.5 to W12,and that of copper nitrate hydroxide decreases and the peak vanishes when more than 6 mL of ammonia was added during the catalyst preparation. Clearly,copper hydroxide replaces copper nitrate hydroxide with higher amount of ammonia and its intensity decreases with the increase of ammonia. The phase identification results of each sample are listed in Table 2.
| Table 2 Phase identification of uncalcined samples determined from the XRD spectra. |
|
Download:
|
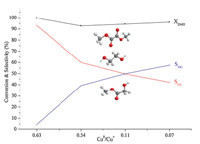
| Fig. 2. Effects of ammonia used on the catalytic performance of each catalyst. (Reaction conditions:p= 2.0 MPa,T= 473 K,H2/DMO = 60,WHSV = 0.65 h -1 .). | |
For a deeper insight into the transformation of the precursor species when suffering from calcination,XRD characterizations of the calcined samples were carried out. As shown in Fig. S3 in Supporting information,all the catalysts possess two phases,a minor or well dispersed chrysocolla phase and a copper oxide phase. It has been reported that chrysocolla derives from the copper tetrammine complex that exchanges with a pair of adjacent silanol groups thus establishes a strong interaction between the copper ions and the support surface structure [26]. Limited by the available silanol groups on the surface,the ion-exchanged copper species contribute only a small part of the copper loading. These species are reported to be of isolated nature and can only be reduced to Cu+ in hydrogen at up to 673 K. The resulted Cu+ species retains this isolated nature for the strong metal-support interaction (MSI). Hence,it is not surprised that only a minor chrysocolla phase was observed.
The species of the calcined samples can also be verified by the FTIR spectra (Fig. S4 in Supporting information,only 400-1000 cm-1 range is given here). The absorption bands at 1106,806 and 473 cm-1 are assigned to different vibration modes of the Si-O bonds in amorphous silica [15, 17],while the broad absorption band between 3600 and 3200 cm-1 attributes to the overlapping of OH stretching of adsorbed water and silanols [27]. The band at 1640 cm-1 corresponds to the bending mode of OH groups of absorbed water [1]. The characteristic band of chrysocolla (also called copper phyllosilicate) locates at about 670 cm-1 (the other characteristic band at 1040 cm-1 being overlapped by the broad absorption band of amorphous silica), while that of copper oxide lies near 575 cm-1 (the other two characteristic bands at 500 and 460 cm-1 overlap with the broad absorption band of amorphous silica) [25, 28, 29]. It can be clearly seen that the peak located at 670 cm-1 is absent in the silica support while that of the catalysts exits with different peak intensities. The relative amount of supported copper phyllosilicate can be evaluated viathe so-calledI670/I800[29],which is the integrated intensity of the δOHband at 670 cm-1 normalized to the integrated intensity of the symmetric vSiO band of amorphous silica at 800 cm-1 . As listed in Table 1,copper phyllosilicate does only account for a very small portion of the copper loading,and the value declines when the ammonia solution volume increases.
However,species after reduction are as the active catalyst. Thus, it is necessary to elucidate the species following reduction. Their XRD spectra are illustrated in Fig. S5 in Supporting information. All samples after reduction exhibit the co-existence of Cu (JCPDS 04-0836) and Cu2O (JCPDS 05-0667). Kohleret al. [26] suggested that two kinds of copper species,namely the precipitated copper hydroxide particles and isolated copper ions,formed in the ionexchanged Cu/SiO2 catalysts. The former was derived from the washing procedure and the latter was generated from the ionexchange process. Copper ions remained isolated while Cu(OH)2 was transformed to CuO particles after calcination. The corresponding copper states after reduction were Cu0 and Cu+ , respectively. Toupance et al. [29],however,offered a different interpretation. They suggested that the two types of supported Cu2+ species formed were grafted Cu2+ ions and copper phyllosilicate. The cationic copper amine complex in solution adsorbed onto the negatively charged silica surface due to the high solution pH value by electrostatic interactions and the grafting took place in the subsequent drying step. Copper phyllosilicate formed in solution through the reaction between silicic acid arising from silica dissolution and [Cu(OH)2(H2O)4] 0 complex. Upon reduction, the grafted Cu2+ ions and copper phyllosilicate were transformed to Cu0 and Cu+ ,respectively.
Finally,in order to better understand the reducibility of the catalysts prepared,TPR characterizations are conducted. As shown in Fig. 1,they present a regular change of the reduction behavior of the catalysts. For W2.5,there is only one wide reduction peak implying that the copper species are well-dispersed. Taking the asymmetry of the peaks into account,at least two peaks can be obtained by deconvolution using the Lorentz method. For W3 to W12,two different peaks are obviously present. Herein,we denote the peak located at low temperature asa-peak and the one at high temperature asb-peak. Similarly,Wanget al. [13] assigned these two peaks of Cu/SiO2prepared by the DP method using ammonia as a precipitant to the transformation of Cu2+ to Cu+ and reduction of Cu+ to Cu0 ,respectively. 3.2. Activity test
Activity test (Fig. 2) of DMO hydrogenation displays that the conversion of DMO changes insignificantly with the amount of ammonia under the reaction conditions (473 K,2 MPa,n(H2)/ n(DMO) = 60,WHSV = 0.65 h-1 ),while the selectivity of EG declines and that of MG increases dramatically. For sample W2.5,on which side reactions took place most,the selectivities for EG,MG and side products (ethanol,PDO,BDO) were 93.13%, 4.54% and 2.34%,respectively. For sample W12,which had the least side products generated,the corresponding selectivities were 42.20%,57.47% and 0.33%. Thus the decrease in the selectivity of EG is not caused by side reactions. 3.3. Discussion
From the XRD spectra of the uncalcined samples,it is noted that the chrysocolla phase formed among all the catalysts. With the increase in ammonia amount,the other phase changes from the copper nitrate hydroxide to copper hydroxide. Chrysocolla derives from the reaction between silicic acid (viasilica dissolution) and [Cu(OH)2(H2O)4] 0 complex in solution [29]. It has been reported [30] that the labile copper tetramine complex could rapidly exchange. In ammonia solutions,two surface protons of silica gels exchange with one tetramine cupric ion to yield species such as (≡SiO)2Cu(NH3)2and form two ammoninum ions while in acidic solutions,only one proton exchanges with one cupric ion to generate surface species (≡SiO)Cu(H2O)nNO3(1≤n≤3) [31].
Copper hydroxide and copper nitrate hydroxide come from the precipitation of Cu2+ ions with OH - ,which is formed during the dissociation of NH3·H2O. Under different pH values controlled by adding KOH,Cu2+ can form four main hydrolyzed products [32], Cu2(OH)2 2+ ,Cu(OH)2,Cu(OH)3 - and Cu(OH)4 2- .Cu2+ dominates in solution when the pH value is below 5,and between the pH range of 7-10,Cu2(OH)2 2+ is the main product. At high pH values (>10), the other three products appear sequentially and become the main components. In an ammonical environment,copper nitrate hydroxide is reported to form at pH 5.0 [33] and Cu2+ converts to copper hydroxide at pH 7 while at more elevated pH levels (10- 11) copper tetramine dominates [34]. From the XRD spectra,it is obvious that in relatively low pH environments copper nitrate hydroxide precipitates first. The initial pH value of sample W2.5 is 7.89 and copper nitrate hydroxide and copper ammine complexes form. The complexes can be taken away during washing process, and the amount of copper complexes increase with the elevation of the pH values due to the increased amount of ammonia used. Thus, the copper loading descends with the elevation of the amount of ammonia. The silica used is in the form of ultra fine particles with a diameter of 15±5 nm and its surface is negatively charged. Copper ammine complexes exchanging with two silanol groups of a pair of adjacent ultra fine particles may build large pores. These large pores can be confirmed by the pore distribution of the samples whose pore diameter is at about 32 nm. This may contribute to the enlargement of both the pore volume and the average pore size of all samples compared with the support. Besides,the package of silica particles may also contribute. The copper ammine complexes may absorb onto the negatively charged surface of the silica particlesviaelectrostatic interactions at the high pH values and the portion with sufficiently strong interactions cannot be removed by washing and becomes grafted copper species after dryness [29]. And part of the grafted species on the silica particle surface may block the pores of the silica particles thus the surface area decreases with the increased pH values.
Species in the uncalcined samples like grafted copper species, Cu2(OH)2NO3and Cu(OH)2transform into CuO particles and then convert to Cu0 after reduction. But chrysocolla can only be reduced to Cu+ due to the strong MSI and further reduction of the resulted Cu + requires temperatures higher than 873 K [1]. In the TPR profiles,thea-peak andb-peak are assigned to the transformation of Cu2+ to Cu+ and reduction of Cu+ to Cu0 ,respectively. Thus,the hydrogen consumption of thea-peak attributes to the reduction of CuO particles as well as that of the chrysocolla phase,while the hydrogen consumption of theb-peak results from the reduction of the Cu+ originated from the CuO particles. By comparing the area of the two peaks,we can evaluate the relative amount of copper with the two oxidation states after reduction. Thus,from the TPR profiles,we can see that the relative amount of Cu0 decreases with the increase of ammonia used. The XRD spectra of the reduced samples can also give quantitative information [13]. The diffraction peak intensity of Cu0 becomes weaker with the increased amount of ammonia used,which means less Cu0 exists in the reduced catalysts. The XRD spectra of the calcined samples show that all samples are well crystallized after calcination,but there are no diffraction peaks of CuO phase found in the reduced samples, which means that all the CuO has been transformed under the reduction conditions. Thus,the copper species after reduction is either metalic Cu or Cu2O. This means that the relative amount of Cu2O increases as the amount of Cu0 decrease,thus the ratio Cu0 / Cu + also decreases from W2.5 to W12. Activity test results show that the selectivities of EG and MG are independent of the copper loading but related to the Cu0/Cu+ ratio directly,and catalysts with a relatively large Cu0/Cu+ ratios are in favor of the selectivity of EG. In the present experiment,the addition of 2.5 mL ammonia solution (the molar ratio of NH3and Cu2+ is about 0.9) can produce a better Cu0/Cu+ ratio after reduction and thus W2.5 has a better selectivity of EG. 4. Conclusion
The Cu0/Cu+ ratio of the Cu/SiO2 catalysts prepared by the deposition-precipitation method using ammonia as a precipitant can be modulated by controlling the amount of ammonia used and it may result from the discrepancy of species at different preparation stages.
Chrysocolla forms rapidly in the ammonical solution. It remains during the subsequent drying,calcination processes and is transformed to Cu+ after reduction at 723 K. Copper nitrate hydroxide forms when using 2.5 mL or 3 mL of concentrated ammonia solution to prepare the catalysts,and copper hydroxide precipitates when increasing the volume to 6 mL or 12 mL. They are both transformed into the CuO particles after calcination and then to Cu0 upon reduction. These catalysts have different Cu0/Cu+ ratios. When applied for the hydrogenation of DMO to EG,it is found that the selectivities of EG and MG are independent of the copper loading but related to the Cu0/Cu+ ratio directly. Catalyst prepared using 2.5 mL ammonia solution (n(NH3)/n(Cu2+ ) = 0.9) can produce a better Cu0/Cu+ ratio after reduction and thus W2.5 has a better selectivity of EG. Acknowledgment
Funding for the present study from the National Basic Research Program of China (973 Program,No. 2011CB710800) and the Opening Foundation (2014) of Zhejiang Zanyu Technology Co.,Ltd. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.050.
| [1] | L.F. Chen, P.J. Guo, M.H. Qiao, et al., Cu/SiO2 catalysts prepared by the ammoniaevaporation method: texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol, J. Catal. 257 (2008) 172-180. |
| [2] | H.R. Yue, Y.J. Zhao, X.B. Ma, et al., Ethylene glycol: properties, synthesis, and applications, Chem. Soc. Rev. 41 (2012) 4218-4244. |
| [3] | K. Fujii, M. Matsuda, K. Mizutare, et al. Process for continuously preparing ethylene glycol, US 4453026, 1984. |
| [4] | X.G. Zhao, X.L. Lvy, H.G. Zhao, et al., Study on Pd/a-Al2O3 catalyst for vapor-phase coupling reaction of CO with CH3ONO to (CH3OOC)2, Chin. J. Catal. 25 (2004) 125-128. |
| [5] | L.R. Zehner, R. Warren Lenton, Process for the preparation of ethylene glycol, US 4112245 A, 1978. |
| [6] | U. Matteoli, G. Menchi, M. Bianchi, et al., Selective reduction of dimethyl oxalate by ruthenium carbonyl carboxylates in homogeneous phase Part IV, J. Mol. Catal. 64 (1991) 257-267. |
| [7] | H.T. Teunissen, C.J. Elsevier, Ruthenium catalysed hydrogenation of dimethyl oxalate to ethylene glycol, Chem. Commun. 12 (1997) 667-668. |
| [8] | A.Y. Yin, X.Y. Guo, W.L. Dai, et al., High activity and selectivity of Ag/SiO2 catalyst for hydrogenation of dimethyl oxalate, Chem. Commun. 46 (2010) 4348-4350. |
| [9] | Z. He, H.Q. Lin, P. He, et al., Effect of boric oxide doping on the stability and activity of a Cu/SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol, J. Catal. 277 (2011) 54-63. |
| [10] | S. Zhao, H.R. Yue, Y.J. Zhao, et al., Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: enhanced stability with boron dopant, J. Catal. 297 (2013) 142-150. |
| [11] | A.Y. Yin, C. Wen, X.Y. Guo, et al., Influence of Ni species on the structural evolution of Cu/SiO2 catalyst for the chemoselective hydrogenation of dimethyl oxalate, J. Catal. 280 (2011) 77-88. |
| [12] | B.W.Wang, Q. Xu, H. Song, G.H. Xu, Synthesis of methyl glycolate by hydrogenation of dimethyl oxalate over Cu-Ag/SiO2 catalyst, J. Nat. Gas Chem. 16 (2007) 78-80. |
| [13] | B.W. Wang, X. Zhao, Q. Xu, G.H. Xu, Preparation and characterization of Cu/SiO2 catalyst and its catalytic activity for hydrogenation of diethyl oxalate to ethylene glycol, Chin. J. Catal. 29 (2008) 275-280. |
| [14] | A.Y. Yin, X.Y. Guo, K.N. Fan, W.L. Dai, Ion-exchange temperature effect on Cu/HMS catalysts for the hydrogenation of dimethyl oxalate to ethylene glycol, Chem- CatChem 2 (2010) 206-213. |
| [15] | A.Y. Yin, X.Y. Guo, K.N. Fan, et al., Influence of copper precursors on the structure evolution and catalytic performance of Cu/HMS catalysts in the hydrogenation of dimethyl oxalate to ethylene glycol, Appl. Catal. A 377 (2010) 128-133. |
| [16] | C. Wen, A.Y. Yin, Y.Y. Cui, et al., Enhanced catalytic performance for SiO2-TiO2 binary oxide supported Cu-based catalyst in the hydrogenation of dimethyloxalate, Appl. Catal. A 458 (2013) 82-89. |
| [17] | X.B. Ma, H.W. Chi, H.R. Yue, et al., Hydrogenation of dimethyl oxalate to ethylene glycol over mesoporous Cu/MCM-41 catalysts, AIChE J. 59 (2013) 2530-2539. |
| [18] | J.L. Gong, H.R. Yue, Y.J. Zhao, et al., Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites, J. Am. Chem. Soc. 134 (2012) 13922-13925. |
| [19] | S.R. Wang, X.B. Li, Q.Q. Yin, et al., Highly active and selective Cu/SiO2 catalysts prepared by the urea hydrolysis method in dimethyl oxalate hydrogenation, Catal. Commun. 12 (2011) 1246-1250. |
| [20] | L.M. He, X.C. Chen, J.S. Ma, et al., Characterization and catalytic performance of sol-gel derived Cu/SiO2 catalysts for hydrogenolysis of diethyl oxalate to ethylene glycol, J. Sol-Gel Sci. Technol. 55 (2010) 285-292. |
| [21] | X.Y. Guo, A.Y. Yin, W.L. Dai, et al., One pot synthesis of ultra-high copper contented Cu/SBA-15 material as excellent catalyst in the hydrogenation of dimethyl oxalate to ethylene glycol, Catal. Lett. 132 (2009) 22-27. |
| [22] | S.R. Wang, Q.Q. Yin, X.B. Li, Catalytic performance and texture of TEOS based Cu/ SiO2 catalysts for hydrogenation of dimethyl oxalate to ethylene glycol, Chem. Res. Chin. Univ. 28 (2012) 119-123. |
| [23] | C. Carlini, D.G. Marco, M. Mario, et al., Selective synthesis of isobutanol by means of the Guerbet reaction: Part 2. Reaction of methanol/ethanol and methanol/ ethanol/n-propanol mixtures over copper based/MeONa catalytic systems, J. Mol. Catal. A: Chem. 200 (2003) 137-146. |
| [24] | S. Veibel, J.I. Nielsen, On the mechanism of the Guerbet reaction, Tetrahedron 23 (1967) 1723-1733. |
| [25] | A.Y. Yin, X.Y. Guo, W.L. Dai, et al., The nature of active copper species in Cu/HMS catalyst for hydrogenation of dimethyl oxalate to ethylene glycol: new insights on the synergetic effect between Cu0 and Cu+, J. Phys. Chem. C 113 (2009) 11003-11013. |
| [26] | M.A. Kohler, H.E. Curry-Hyde, A.E. Hughes, et al., The structure of Cu/SiO2 catalysts prepared by the ion-exchange technique, J. Catal. 108 (1987) 323-333. |
| [27] | B. Zhang, S.G. Hui, S.H. Zhang, et al., Effect of copper loading on texture, structure and catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol, J. Nat. Gas Chem. 21 (2012) 563-570. |
| [28] | T. Toupance, M. Kermarec, C. Louis, Metal particle size in silica-supported copper catalysts. Influence of the conditions of preparation and of thermal pretreatments, J. Phys. Chem. B 104 (2000) 965-972. |
| [29] | T. Toupance, M. Kermarec, J.F. Lambert, et al., Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption, J. Phys. Chem. B 106 (2002) 2277-2286. |
| [30] | L. Trouillet, T. Toupance, F. Villain, et al., In situ characterization of the coordination sphere of Cu(Ⅱ) complexes supported on silica during the preparation of Cu/SiO2 catalysts by cation exchange, PCCP 2 (2000) 2005-2014. |
| [31] | H. Tominaga, M. Kaneko, Y. Ono, Cation exchange of surface protons on silica gel with cupric ions, J. Catal. 50 (1977) 400-406. |
| [32] | Y.K. Leong, Yield stress and zeta potential of nanoparticulate silica dispersions under the influence of adsorbed hydrolysis products of metal ions-Cu(Ⅱ), Al(Ⅲ) and Th(IV), J. Colloid Interface Sci. 292 (2005) 557-566. |
| [33] | C.J.G. Van Der Grift, P.A. Elberse, A. Mulder, et al., Preparation of silica-supported copper catalysts by means of deposition-precipitation, Appl. Catal. 59 (1990) 275-289. |
| [34] | M.A. Kohler, J.C. Lee, D.L. Trimm, et al., Preparation of Cu/SiO2 catalysts by the ionexchange technique, Appl. Catal. 31 (1987) 309-321. |