Graphene [1, 2, 3],an allotrope of carbon,has engendered tremendous attention from the scientific community. As a twodimensional single-layer sheet of sp 2 -bonded carbon,graphene is the mother of all graphitic forms,including zero-dimensional fullerenes,one-dimensional carbon nanotubes,and three-dimensional graphite. Compared with other graphitic materials,graphene shows many outstanding advantages,such as remarkable thermal and chemical stability,good mechanical strength,and high surface-to-weight ratios. Moreover,with a large delocalized π-electron system,graphene can form a strong p-pstacking interaction with some organic molecules. Therefore,graphene can be expected to serve as a promising adsorbent for some analytes. Recently,graphene and graphene-based materials have been synthesized and successfully applied for sample pretreatment in solid phase extraction (SPE) [4, 5],magnetic solid phase extraction (MSPE) [6, 7],and solid phase microextraction (SPME) [8, 9].
SPME,first developed by Pawliszyn and coworkers in the early 1990s [10],has been widely used as an effective sample pretreatment technique. It has advantages including almost no consumption of organic solvent,simplicity of operation,and easy linkup with gas chromatography. The mechanism of SPME is based on the equilibrium of the analytes between the sample and the fiber coating [11]. Thus,the adsorbent material coated onto the fiber is critical in improving the SPME performance. Although a number of SPME coatings are commercially available,their performance is not always satisfactory for the extraction of some analytes,especially from complex samples because of their instability at high temperatures and the limited selectivity of the coatings [12].
To overcome these problems,various novel SPME fiber coatings, including polymeric ionic liquid material [13, 14, 15],metal organic framework material [16, 17],mesoporous material [18],and carbon nanomaterial [19, 20],have been explored. Among all the coating materials,graphene can possibly become an ideal choice due to its large specific area and excellent π-π electrostatic stacking property. However,up to now,there have only been a few reports about the application of graphene on the fiber coating for SPME. In this regard,Chenet al.[21] first prepared a graphene-coated SPME fiber for the extraction of pyrethroid pesticides from water samples with satisfactory results. Then,graphene-based SPME coatings were reported to extract organochlorine pesticides [22, 23],polybrominated diphenyl ethers [24],triazine herbicides [25],and polycyclic aromatic hydrocarbons [26] from water and soil samples. It is evident that further explorations of the potential applications of the graphene composite-based SPME fibers are necessary.
Nitrobenzene compounds are widely used in chemical industry. In recent years,the release of nitrobenzene compounds into environmental water or soil samples has drawn considerable attention due to their toxicity,persistence,and accumulation in food chains [27, 28]. Therefore,developing rapid,sensitive,and effective methods for the trace analysis of nitrobenzene compounds in environmental samples is desirable.
In the current study,graphene was chosen as the extraction material and was coated on a stainless steel wire through sol-gel technique for the SPME. The applicability of the graphene composite-coated fiber for SPME was investigated through the extraction of five nitrobenzene compounds from water and soil samples followed by analysis with gas chromatography-mass spectrometry (GC-MS). 2. Experimental 2.1. Chemicals and materials
Standards of the nitrobenzene compounds (nitrobenzene, 2-nitrotoluene,4-nitrotoluene,4-nitrochlorobenzene and 2-nitrochlorobenzene) were purchased from Agricultural Environmental Protection Institution of Tianjin (Tianjin,China). A mixture stock solution containing each of the five nitrobenzene compounds at 50.0 mg/L was prepared in acetone and stored at 4℃.
Dichloromethane (CH2Cl2),hydrofluoric acid (HF,40%,w/w), methyltrimethoxysilane (MTMOS),trifluoroacetic acid (TFA,99%) and hydroxy terminated polydimethylsiloxane (HO-PDMS) were obtained from Boaixin Chemical Reagents Company (Baoding, China). Sodium chloride (NaCl) was purchased from Tianjin Fuchen Chemical Reagent Factory (Tianjin,China). Stainless steel wires (o.d. 150mm) were purchased from Anting Micro-Injector Factory (Shanghai,China). The commercial SPME fibers coated with polydimethylsiloxane (PDMS,100mm),carbowax/divinylbenzene (CW/DVB,70mm),and polyacrylate (PA,85mm) were purchased from Supelco (Bellefonte,PA,USA). Graphene was synthesized according to the method reported in our previous work [29]. 2.2. Preparation of graphene composite-coated SPME fiber
Based on the literature method [24] with some modifications, the fabrication of the graphene composite-coated fiber involved the following steps. Firstly,the sol solution of graphene composite coating material was prepared as follows: Graphene (50 mg), CH2Cl2(100mL),MTMOS (100mL),and HO-PDMS (100mL) were added to an Eppendorf tube (1.0 mL) and mixed by vortexing for 5 min. Then,80mL of TFA (95% water solution) was added and mixed thoroughly for 2 min. Secondly,a stainless steel wire,which was first etched with HF solution for 15 min,was dipped into the above sol solution to a depth of 1.0 cm for 2 min. Then,it was pulled out and dried at room temperature for 10 min so that the gel coating was formed on the etched surface. This procedure was repeated about 12 times so as to obtain a desired thickness of the coating (about 50mm). The fiber was then assembled into a 5mL GC microsyringe by replacing the plunger with a homemade SPME device,and finally it was conditioned in the GC injector under nitrogen at 200℃ for 2 h. 2.3. Instrumental and analytical conditions
The DF-101S temperature-controlled magnetic stirrer was purchased from Baoding High-tech Zone Sunshine Science Instrument Company (Baoding,China). The WH-861 vortex shaker was from Shanghai Jinggong Industrial Limited Liability Company (Shanghai,China).
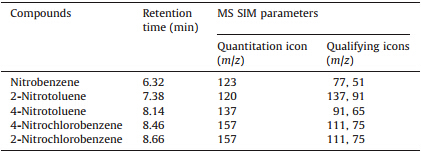
A Shimadzu (Kyoto,Japan) GCMS-QP2010 SE system equipped with a TG-5MS fused silica capillary column (30 m×0.25 mm× 0.25mm) (Scientific,Thermo,www.thermo.com/columns) was used for analysis. The injector was operated in the splitless mode, and the injection port temperature was maintained at 270℃. Chromatographic separations were performed with an initial oven temperature of 60℃,followed by heating at 10℃/min to 150℃ (held for 2 min),and finally programmed at 30℃/min to 210℃. The mass spectrometer was operated in the electron ionization (EI) mode with the temperature of the transfer line and the ion source being kept at 250℃ and 200℃,respectively. Data collection was carried out in the selected ion monitoring (SIM) mode. Ion peaks including one for quantification and two for qualification were monitored for each nitrobenzene compound based on the full-scan results. The retention time and SIM parameters for each analyte are listed in Table 1. The quantification was performed by calculating the absolute peak areas in SIM mode. Helium at a flow rate of 37.5 cm/s in the constant linear velocity mode was employed as the carrier gas.
| Table 1 Retention time and SIM parameters for each analyte. |
Lake water was collected from our campus (Baoding,China); river water was collected from Jiucheng (Huanghua,China). For headspace SPME,15.0 mL of water sample and 5.5 g of NaCl were added into a 30 mL glass vial and then mixed until the NaCl was completely dissolved.
Soil samples were collected both from our campus (Baoding, China) and from a farmland (Zhongjie,China),respectively,which were pulverized and passed through 450mm sieves. 5.0 g soil sample was weighted into a 30.0 mL glass vial. Then,15.0 mL saturated NaCl solution (about 37%,w/v) was added and mixed thoroughly to form a homogeneous soil suspension for headspace SPME. 2.5. Headspace SPME procedure The 30.0 mL glass vial containing 15.0 mL of the above sample solution with a Teflon-lined cap was immersed in a water bath at 50℃. The extraction was performed by the exposure of the graphene composite fiber to the headspace above the sample under stirring at 600 rpm for 30 min. After extraction,the SPME fiber was inserted into the GC injector to desorb the analytes at 270℃ for 1.5 min for GC-MS analysis. 3. Results and discussion 3.1. Surface structure of the graphene composite-coated SPME fiber Fig. 1A shows the low-magnification surface morphological structure of the graphene composite-coated fiber observed by scanning electron microscope (SEM),from which it can be seen that the surface of the coating is rough and porous. The sectional image of the fiber shows that the thickness of the coating is approximately 50mm. The SEM image with high-magnification (Fig. 1B) shows a porous and wrinkled surface structure,which would significantly increase the surface area available on the fiber and improve the kinetics of the extraction process and extraction efficiency.

|
Download:
|
| Fig. 1. Scanning electron micrographs of graphene composite fiber coating. (A) 200×magnification; (B) 1000×magnification. | |
|
Download:
|
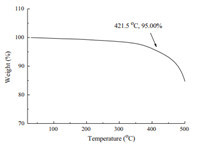
| Fig. 2. Thermogravimetric curve of the graphene composite coating. | |
Compared with silica fibers,the mechanical strength of the graphene composite fiber was greatly improved when stainless steel wire was used as the support,which could prevent the coated fiber from being broken during stirring and injection. In this study, the stability of the graphene composite fiber was examined by investigating the lifetime of the fiber. By using the same fiber,there is no obvious difference in extraction efficiency after 20,50,100,or 160 extractions for the five nitrobenzene compounds,which indicates that the graphene composite-coated fiber has good stability. 3.3. Optimization of headspace SPME method
For the extraction of the semivolatile and volatile compounds, headspace SPME mode can offer a high sensitivity,minimize the interference from sample matrix and prolong the lifetime of the fiber [30]. Therefore,in this work,the SPME of the five nitrobenzene compounds was carried out under headspace mode. In order to achieve the best extraction efficiency towards the five nitrobenzene compounds,extraction temperature,extraction time,salt concentration,stirring rate,headspace volume and desorption time were investigated. 3.3.1. Extraction temperature and extraction time
For headspace SPME,optimization of extraction temperature is very important,especially for semivolatile compounds such as nitrobenzenes. In the current study,the extraction temperatures ranging from 20℃to60℃ were investigated. As shown in Fig. 3A, the peak areas for the analytes were increased when the extraction temperature was increased from 20℃to50℃,and then decreased. The reason for this could be that the increase of the extraction temperature can enhance the mass transfer of the analytes from sample to the headspace and further to the fiber sorbent,which leads to the increase of the extraction efficiency. However,too high a temperature may decrease the amount of the analytes adsorbed on the fiber. Based on the above experimental result,the extraction temperature at 50℃ was chosen for the experiments.

|
Download:
|
| Fig. 3. Effect of extraction conditions headspace-SPME efficiency. (A) Effect of extraction temperature; (B) effect of extraction time; (C) effect of salt concentration; (D) effect of stirring rate; (E) effect of volume ratio; (F) effect of desorption time. | |
Extraction time is also a key parameter that influences the extraction performance of the method. In this work,the effect of extraction time was examined in the range from 10 min to 40 min. Fig. 3B displays that the peak areas increased with the extraction time being increased from 10 min to 25 min and then remained almost unchanged. This phenomenon indicated that the adsorption was a dynamic equilibration process,and once the equilibrium was achieved,a further extension of the extraction time had almost no effect on the extraction efficiency. Therefore,the extraction time of 25 min was selected. 3.3.2. Salt concentration
The addition of salt to sample solution is favorable for headspace SPME since it can enhance the transfer of analytes from sample matrix to headspace due to salting-out effect. Thus, sample solutions with different concentrations of NaCl (0%,10%, 20%,30% and 37% (w/v)) were investigated to assess the influence of the concentration of salt on the extraction efficiency for the analytes. The result shown in Fig. 3C indicated that the maximum extraction efficiency for the analytes was achieved when the concentration of NaCl was 37%,namely,when NaCl was saturated in the sample solution. Therefore,subsequent experiments were performed with saturated NaCl in the sample solution (about 37%). 3.3.3. Stirring rate
Stirring rate affects the extraction performance in SPME. Generally,the larger the agitation rate is,the faster the mass transfer between the sample matrix and the headspace will be. The effect of stirring rate was studied in the range from 200 rpm to 800 rpm. From the obtained results (shown in Fig. 3D),it can be seen that the peak areas were increased with the stirring rate being increased from 200 min to 500 rpm and then remained almost unchanged. Therefore,a stirring rate of 600 rpm was selected. 3.3.4. Effect of volume ratio
The volume ratio between the headspace and sample solution influences the extraction efficiency of the graphene compositecoated fiber for the compounds. Headspace SPME is based on the fact that in a sealed vessel,the volatile solutes will establish a distribution equilibrium between the headspace and sample solution (water solution or soil suspension). The optimization experiment was conducted by changing the ratio of the headspace to sample volume from 1:4 to 3:2,namely,changing the volume of sample solution from 24.0 mL to 12.0 mL. Fig. 3E illustrates that the peak areas of the analytes increased when the volume ratio was increased from 1:4 to 1:1,and then decreased gradually. Therefore, the volume ratio of 1:1 (15.0 mL sample solution) was chosen for the experiment. 3.3.5. Desorption time
A proper desorption time can lead to an effective desorption of the analytes from the graphene composite-coated fiber. In this study,desorption time was investigated at 270℃ from 0.5 min to 3.0 min. The result (shown in Fig. 3F) revealed that there was no significant difference for the peak areas of the analytes after desorption at 270℃ for 1.5 min and also no carry-over was observed for the five nitrobenzene compounds after 1.5 min. Thus, desorption at 270℃ for 1.5 min was chosen. 3.4. Comparison with commercial fibers
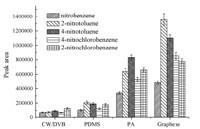
The graphene composite-coated fiber was compared with the commercial PDMS (100mm),CW/DVB (70mm) and PA (85mm) fibers for the extraction of the analytes. As shown in Fig. 4,the extraction efficiency of the graphene composite fiber was much higher than those of the other three commercial fibers. The higher extraction efficiency of the home-made fiber toward the nitrobenzene compounds may be attributed to the porous and wrinkled structure of the graphene composite fiber coating,which could increase the available adsorption sites. Furthermore,graphene could interact with the nitrobenzene compounds more strongly than the others since graphene possesses large delocalized pelectron system,which can form a strongp-stacking interaction with the aromatic rings of the nitrobenzene compounds.
|
Download:
|
| Fig. 4. Comparison with commercial fibers for the extraction efficiency of nitrobenzene compounds. | |
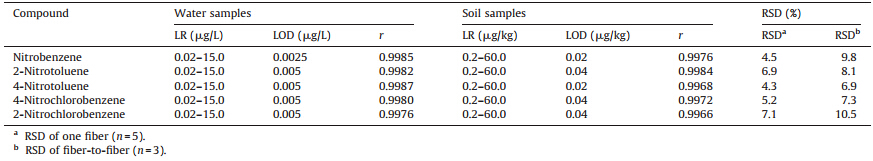
Under the optimized conditions,the linear range (LR),the limits of detection (LODs),the correlation coefficients (r) and relative standard deviations (RSDs) were evaluated to validate the developed headspace SPME-GC-MS method. The linear regression analysis was performed using peak areas against the concentrations of the analytes. For water samples,a series of standard solutions containing each of the nitrobenzene compounds at seven concentrations,i.e.,0.02,0.05,0.20,2.0,10.0,15.0 and 18.0 mg/L were prepared for the establishment of the calibration curve. For soil samples,non-polluted soil was used as blanks for matrixmatched standard calibrations. An appropriate amount of the mixture standard solution of the analytes was added to prepare a series of standard soil samples containing each of the five nitrobenzene compounds at seven concentrations of 0.2,0.25, 1.0,10.0,50.0,60.0 and 75.0mg/kg,respectively. For each level,five replicate analyses were performed. The results summarized in Table 2 show that the linearity existed in the range of 0.02- 15.0mg/L for water samples and 0.2-60.0mg/kg for soil samples, withrvalues of 0.9976-0.9987 and 0.9966-0.9984,respectively. The LODs were 0.0025-0.005mg/L for water samples and 0.02- 0.04mg/kg for soil samples. The same fiber was used for five replicate extractions under the same conditions and the RSD was below 7.1%. The RSDs for fiber-to-fiber variations were less than 10.5% using three different fibers prepared in the same way.
| Table 2 Linear range (LR),limits of detections (LODs) and correlation coefficients (r) in water and soil samples. |
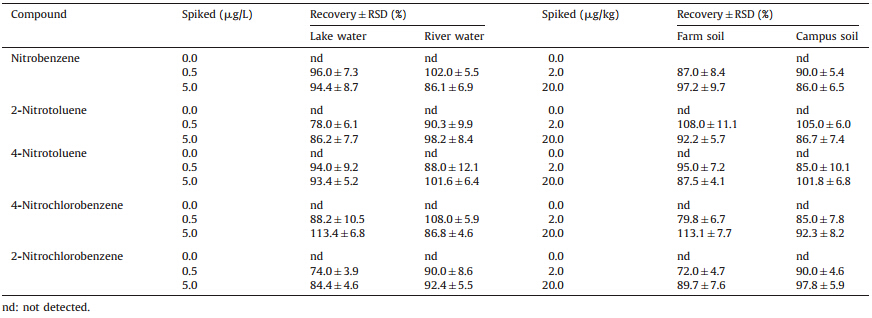
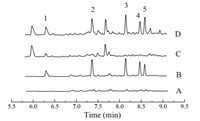
The graphene composite fiber-based headspace-SPME-GC-MS method was further evaluated for the determination of the five nitrobenzene compounds in real water and soil samples. The results showed that no nitrobenzene compounds were detected in water samples while nitrobenzene was found to be 0.06mg/kg in the farmland soil sample. The recoveries of the method were determined to test the accuracy of the method. The water samples were spiked with the standards of the analytes at the concentrations of 0.5 and 5.0mg/L and the soil samples were spiked at 2.0 and 20.0mg/kg,respectively. As a result,the recoveries of the five nitrobenzene compounds from the samples are in the range from 72.0% to 113.2% with the RSDs less than 12.1% (Table 3),which demonstrates that the accuracy and precision of the present method are acceptable. Fig. 5 shows the typical GC-MS chromatograms of the lake water and soil samples.
| Table 3 Recoveries and precisions (RSD,n=5) of the five nitrobenzene compounds in water and soil samples obtained by headspace SPME-GC-MS method. |
|
Download:
|
| Fig. 5. The chromatograms of lake water (A),the lake water spiked at the concentration of 0.5mg/L each of the nitrobenzene compounds (B),the farmland soil (C) and the soil sample spiked at 2.0mg/kg each of the five nitrobenzene compounds (D). Peak identification: (1) nitrobenzene,(2) 2-nitrotoluene,(3) 4-nitrotoluene,(4) 4-nitrochlorobenzene,(5) 2-nitrochlorobenzene. | |
In the present study,a graphene composite-coated fiber has been preparedviasol-gel technology and used as SPME sorbent to extract five nitrobenzene compounds from environmental water and soil samples for GC-MS analysis. The fiber coating had a rough, porous and wrinkled structure,and the graphene composite fiber had a good thermal stability,high extraction efficiency,and long lifetime. Acknowledgments
Financial support from the National Natural Science Foundation of China (No. 31171698),the Innovation Research Group Program of Department of Education of Hebei for Hebei Provincial Universities (No. LJRC009) and the Natural Science Foundation of Hebei Province (No. B2012204028) are gratefully acknowledged.
| [1] | L. Saghatforoush, M. Hasanzadeh, N. Shadjou, Polystyrene-graphene oxide modified glassy carbon electrode as a new class of polymeric nanosensors for electrochemical determination of histamine, Chin. Chem. Lett. 25 (2014) 655-658. |
| [2] | C. Wang, S. de Rooy, C.F. Lu, et al., An immobilized graphene oxide stationary phase for open-tubular capillary electrochromatography, Electrophoresis 34 (2013) 1197-1202. |
| [3] | C. Feng, H.Y. Zhang, N.Z. Shang, S.T. Gao, C. Wang, Magnetic graphene nanocomposite as an efficient catalyst for hydrogenation of nitroarenes, Chin. Chem. Lett. 24 (2013) 539-541. |
| [4] | Z.H. Wang, Q. Han, J.F. Xia, et al., Graphene-based solid-phase extraction disk for fast separation and preconcentration of trace polycyclic aromatic hydrocarbons from environmental water samples, J. Sep. Sci. 36 (2013) 1834-1842. |
| [5] | H. Zhang, W.P. Low, H.K. Lee, Evaluation of sulfonated graphene sheets as sorbent for micro-solid-phase extraction combined with gas chromatography-mass spectrometry, J. Chromatogr. A 1233 (2012) 16-21. |
| [6] | X.X. Ma, J.T. Wang, M. Sun, et al., Magnetic solid-phase extraction of neonicotinoid pesticides from pear and tomato samples using graphene grafted silica-coated Fe3O4 as the magnetic adsorbent, Anal. Methods 5 (2013) 2809-2815. |
| [7] | W.N. Wang, R.Y. Ma, Q.H. Wu, C. Wang, Z. Wang, Magnetic microsphere-confined graphene for the extraction of polycyclic aromatic hydrocarbons from environmental water samples coupled with high performance liquid chromatographyfluorescence analysis, J. Chromatogr. A 1293 (2013) 20-27. |
| [8] | L.L. Xu, J.J. Feng, J.B. Li, X. Liu, S.X. Jiang, Graphene oxide bonded fused-silica fiber for solid-phase microextraction-gas chromatography of polycyclic aromatic hydrocarbons in water, J. Sep. Sci. 35 (2012) 93-100. |
| [9] | J. Zou, X.H. Song, J.J. Ji, et al., Polypyrrole/graphene composite-coated fiber for the solid-phase microextraction of phenols, J. Sep. Sci. 34 (2011), 2765-2772. |
| [10] | C.L. Arthur, J. Pawliszyn, Solid phase microextraction with thermal desorption using fused silica optical fibers, Anal. Chem. 62 (1990) 2145-2148. |
| [11] | D. Vuckovic, High-throughput solid-phase microextraction in multi-well-plate format, TrAC Trends Anal. Chem. 45 (2013) 136-153. |
| [12] | A. Mehdinia, M.O. Aziz-Zanjani, Recent advances in nanomaterials utilized in fiber coatings for solid-phase microextraction, TrAC Trends Anal. Chem. 42 (2013) 205-215. |
| [13] | J.J. Feng, M. Sun, L.L. Xu, et al., Novel double-confined polymeric ionic liquids as sorbents for solid-phase microextraction with enhanced stability and durability in high-ionic-strength solution, J. Chromatogr. A 1268 (2012) 16-21. |
| [14] | J. Gonzalez-Alvarez, D. Blanco-Gomis, P. Arias-Abrodo, et al., Analysis of beer volatiles by polymeric imidazolium-solid phase microextraction coatings: synthesis and characterization of polymeric imidazolium ionic liquids, J. Chromatogr. A 1305 (2013) 35-40. |
| [15] | J. Jia, X.J. Liang, L.C. Wang, et al., Nanoporous array anodic titanium-supported copolymeric ionic liquids as high performance solid-phase microextraction sorbents for hydrogen bonding compounds, J. Chromatogr. A 1320 (2013) 1-9. |
| [16] | N. Chang, Z.Y. Gu, H.F. Wang, X.P. Yan, Metal organic-framework-based tandem molecular sieves as a dual platform for selective microextraction and highresolution gas chromatographic separation of n-alkanes in complex matrixes, Anal. Chem. 83 (2011) 7094-7101. |
| [17] | L.Q. Yu, X.P. Yan, Covalent bonding of zeolitic imidazolate framework-90 to functionalized silica fibers for solid-phase microextraction, Chem. Commun. 49 (2013) 2142-2144. |
| [18] | X.Z. Du, Y.R. Wang, X.J. Tao, H.L. Deng, An approach to application of mesoporous hybrid as a fiber coating of solid-phase microextraction, Anal. Chim. Acta 543 (2005) 9-16. |
| [19] | N. Rastkari, R. Ahmadkhaniha, N. Samadi, A. Shafiee, M. Yunesian, Single-walled carbon nanotubes as solid-phase microextraction adsorbent for the determination of low-level concentrations of butyltin compounds in seawater, Anal. Chim. Acta 662 (2010) 90-96. |
| [20] | N. Rastkari, R. Ahmadkhaniha, M. Yunesian, L. Baleh, A. Mesdaghinia, Sensitive determination of bisphenol A and bisphenol F in canned food using a solid-phase microextraction fibre coated with single-walled carbon nanotubes before GC/MS, Food Addit. Contam. 27 (2010) 1460-1468. |
| [21] | J.M. Chen, J. Zou, J.B. Zeng, et al., Preparation and evaluation of graphene-coated solid-phase microextraction fiber, Anal. Chim. Acta 678 (2010) 44-49. |
| [22] | Y.B. Luo, B.F. Yuan, Q.W. Yu, Y.Q. Feng, Substrateless graphene fiber: a sorbent for solid-phase microextraction, J. Chromatogr. A 1268 (2012) 9-15. |
| [23] | V.K. Ponnusamy, J.F. Jen, A novel graphene nanosheets coated stainless steel fiber for microwave assisted headspace solid phase microextraction of organochlorine pesticides in aqueous samples followed by gas chromatography with electron capture detection, J. Chromatogr. A 1218 (2011) 6861-6868. |
| [24] | H. Zhang, H.K. Lee, Plunger-in-needle solid-phase microextraction with graphene- based sol-gel coating as sorbent for determination of polybrominated diphenyl ethers, J. Chromatogr. A 1218 (2011) 4509-4516. |
| [25] | Q.H. Wu, C. Feng, G.Y. Zhao, C. Wang, Z. Wang, Graphene-coated fiber for solidphase microextraction of triazine herbicides in water samples, J. Sep. Sci. 35 (2012) 193-199. |
| [26] | S.L. Zhang, Z. Du, G.K. Li, Layer-by-layer fabrication of chemical-bonded graphene coating for solid-phase microextraction, Anal. Chem. 83 (2011) 7531-7541. |
| [27] | X. Li, J.M. Chen, L.C. Du, Analysis of chloro- and nitrobenzenes in water by a simple polyaniline-based solid-phase microextraction coupled with gas chromatography, J. Chromatogr. A 1140 (2007) 21-28. |
| [28] | X.T. Peng, X. Zhao, Y.Q. Feng, Preparation of phenothiazine bonded silica gel as sorbents of solid phase extraction and their application for determination of nitrobenzene compounds in environmental water by gas chromatography-mass spectrometry, J. Chromatogr. A 1218 (2011) 9314-9320. |
| [29] | Q.H. Wu, G.Y. Zhao, C. Feng, C. Wang, Z. Wang, Preparation of a graphene-based magnetic nanocomposite for the extraction of carbamate pesticides from environmental water samples, J. Chromatogr. A 1218 (2011) 7936-7942. |
| [30] | M. Tankiewicz, C. Morrison, M. Biziuk, Application and optimization of headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography- flame-ionization detector (GC-FID) to determine products of the petroleum industry in aqueous samples, Microchem. J. 108 (2013) 117-123. |