b Institute of Polymer Science, DSAPM Lab, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, China;
c School of Media and Communication, Shenzhen Polytechnic, Shenzhen 518055, China
Recently,photopolymerization reactions have received revitalized interest as they congregate a wide range of economic and ecological anticipations,while photoinitiators (PIs) or photoinitiating systems (PISs) h ave been the subject of intense studies [1, 2, 3, 4, 5, 6, 7, 8, 9]. However,the light attenuation is a major issue when the photopolymerization is used for the synthesis of photo-screened functional materials. Hence,we recently proposed a redox photopolymerization using a photocaged base and a peroxide, which represents a new free radical PIS [10, 11, 12, 13]. The main advantages of the PISs are their longeval active centers and high initiating capability. Remarkably,this PIS leads to significant post conversion due to the persistent interactions of the photogenerated amines with the peroxide. Through this mechanism,the problem of light attenuation that is associated with the conventional free radical photopolymerization has been overcome. This finding may enable the use of PIS as a phototrigger for self-propagating polymerization reactions to access a larger number of opaque composites.
As part of our continuous interest in developing highly effective photocaged bases,herein we design a thioxanthonebasedN-phthalimidoamino acid ammonium salt (thioxanthenDBU,Scheme 1),which has taken the following points into consideration. Firstly,the synthesis of thioxanthone (TX) derivatives has recently received interest in photochemistry because of their good absorption characteristics and high photoinitiation efficiency in the visible region [14, 15, 16, 17, 18, 19]. This is significant because visible light is cheap,safe,and able to penetrate formulations consisting of UV absorbing monomers,pigments, and substrates. Secondly,there are significant interactions between N-substituted maleimides and type II PIs,which lead to enhanced photoefficiency [20, 21],thus we would design the new chemicalbonded photosensitive groups comprising of the parentN-phthalimidoamino acids and type II chromophores. Finally,both carboxylic acid derivatives of TXs and photolatent amine have been reported to serve as hydrogen bond donors [22].
In the paper,the aim is to establish the mechanism of the primary photocleavage of thioxanthen-DBU and to facilitate the design of future photocaged bases. In order to obtain the clear-cut information on the redox photopolymerization,we extensively studied the photopolymerization of trimethylol propane triacrylate (TMPTA) in the presence of a latent redox initiator combination composed of thioxanthen-DBU and dibenzoyl peroxide (BPO), and the high efficiency has been demonstrated by real-time RTIR measurements. 2. Experimental
Thisosalicyilic acid (99%),trans-4-(aminomethyl)cyclohexanecarboxylic acid (98%),polyphosphoric acid (85%),acetic anhydride, 1,8-diazabicyclo[5.4.0] undec-7-ene (DBU,99%),and dibenzoyl peroxide (BPO) were purchased from Aladdin-reagent (China). BPO was purified by dissolving the commercial material in CHCl3at room temperature and precipitating by adding an equal volume of MeOH. Trimethylol propane triacrylate (TMPTA,Sartomer Company),isopropyl thioxanthone (ITX,IHT Group) were used as received. All other chemicals used were of analytical grade and used without further purification.
The NMR spectra were obtained on a Varian 300 MHz spectrometer using DMSO-d6and TMS as the solvent and internal standard,respectively. Elemental analysis was obtained on an Elementar Vario EL analyzer. Electrospray ionization mass spectra (ESI-MS) were acquired on a Thermo Finnigan LCQ DECA XP ion trap mass spectrometer,equipped with an ESI source. UV-vis absorption spectra were obtained on a Perkin Elmer Lambda 750 UV-visible spectrophotometer. Acrylate conversions were monitored by realtime Fourier transform infrared (RTIR) spectroscopy using a modified Nicolet 5700 spectrometer. Photopolymerization reactions were conducted in a mold from two glass plates and spacers with 15±1 mm in diameter and 1.2±0.1 mmin thickness,changes in the peak area from 6104 to 6222 cm-1 attributed to the stretching vibration were used to monitor the acrylate polymerization kinetics.
Synthesis route for thioxanthen-DBU was given in Scheme 1.
2-(1,3-Dioxoisoindolin-4-ylthio)benzoic acid (e) [23]: Thiosalicylic acid (15.4 g,0.1 mol) in NaOH aqueous solution (200 mL, 1 mol/L) was warmed until the solid dissolved,and then continually warmed to remove water. Ethanol was added into the sticky mixture,and then the white precipitates were collected by filtration,washed with ethanol,and dried invacuoto obtain sodium thiosalicylate (f). A mixture of f(18.215 g,0.1034 mol),3-nitrophthalimide (15.892 g,0.0827 mol),and DMF (200 mL) was stirred at 80℃ for 8 h. A HCl solution (200 mL,2 mol/L) was added. The precipitate was filtered,washed with water,dried invacuo, and then recrystallized from dioxane to give a yellow powder. Yield: 95.4%. 1HNMR (300 MHz,DMSO-d6):δ 7.29-7.31 (m,2H), 7.47 (m,2H),7.66 (m,2H),7.86 (m,1H). Anal. Calcd. for C15H9NO4S: C,60.19; H,3.03; N,4.68; S,10.71; Found: C,59.48; H,3.20; N,4.54; S,10.51.
Thiochromeno[2,3-e]isoindole-1,3,6(2H)-trione (d) [23]: A suspension of e(6 g,0.02 mol) in polyphosphoric acid (100 g,0.30 mol) was stirred at 150℃ for 90 min,and then the mixture was diluted to 500 mL with ice water. The precipitate was filtered,washed several times with H2O,and dried invacuo. Recrystallisation from xylene gave a yellow powder. Yield: 56.2%. 1HNMR (300 MHz,DMSO-d6):δ 7.63 (t,1H),7.83 (t,1H),7.91 (d,1H),7.95 (d,1H),8.47 (d,1H),8.80 (d, 1H). Anal. Calcd. for C15H7NO3S: C,64.05; H,2.51; N,4.98; S,11.40; Found: C,64.91; H,2.81; N,4.92; S,11.23.
9-Oxo-9H-thioxanthene-3,4-dicarboxylic acid (c) [23]: Compound d(0.55 g,1.95 mmol) in NaOH aqueous solution (60 mL, 0.1 mol/L) was refluxed for 90 min,and then acidified with concentrated HCl. The mixture was refluxed with stirring for 18 h. The crude diacidcwas filtered,washed with water,dried in vacuoto give a yellow powder. Yield: 56.2%. 1HNMR (300 MHz, DMSO-d6):δ 7.66 (t,1H),7.85 (t,1H),8.01 (d,1H),8.10 (d,1H),8.48 (d,1H),8.90 (d,1H). Anal. Calcd. for C15H8O5S: C,60.12; H,2.75; S, 10.58; Found: C,64.91; H,2.81; N,4.92; S,11.23.
9-Oxo-9H-Thioxanthene-3,4-anhydride (b) [23]: A mixture ofc (0.58 g,0.0017 mol) and acetic anhydride (8.5 mL,0.09) in xylene (80 mL) was heated to reflux for 90 min. The precipitated powder was filtered,washed with water,dried invacuoto give a yellow powder b. Yield: 87.3%. 1HNMR (300 MHz,DMSO-d6): δ 7.67 (t, 1H),7.86 (t,1H),8.02 (d,1H,),8.12 (d,1H),8.49 (d,1H),8.92 (d,1H). Anal. Calcd. for C15H6O4S: C,63.83; H,2.14; S,11.36; Found: C, 63.63; H,2.04; S,11.16.
4-((1,3,6-Trioxothiochromeno[2,3-e]isoindol-2(1H,3H,6H)-yl)-methyl)cyclohexanecarboxylic acid (a): A solution of b(5 mmol) and trans-4-(aminomethyl)-cyclohexanecarboxylic acid (0.785 g, 5 mmol) in acetic acid (20 mL) was refluxed for 3 h. The reaction mixture was cooled to room temperature,poured into ice cooled water (50 mL),and stirred for 15 min. A white crystalline product was obtained,filtered and dried. Yield: 66.1%. ESI-MS (negative mode):m/z 420.1 (M-H - ) (calcd. for M-H - : 420.1). 1 H NMR (300 MHz,DMSO-d6):δ 1.05 (m,2H),1.23 (m,2H),1.73 (m,3H),1.90 (m,2H),2.14 (m,1H),3.44 (d,2H),7.63 (t,1H),7.82 (t,1H),7.95 (t, 2H),8.44 (d,1H),8.80 (d,1H). Anal. Calcd. for C23H19NO5S: C,65.54; H,4.54; N,3.32; S,7.61; Found: C,64.34; H,4.59; N,3.27; S,7.46.
4-((1,3,6-Trioxothiochromeno[2,3-e]isoindol-2(1H,3H,6H)-yl)-methyl)cyclohexanecarboxylic acid DBU salt (thioxanthen-DBU): To a(5 mmol) in dioxane (30 mL) was slowly added excess DBU (10 mmol) in dioxane (20 mL) and the mixture was stirred at room temperature for 24 h. The mixture was poured into water (100 mL), filtered,washed thrice with water,and dried to give the white powder. The product can be further purified by silica gel column chromatography using hexane: EtOAc (1:5) to give a pure product. Yield: 85%. ESI-MS (negative mode):m/z420.1 (M-H - ) (calcd. for M-H - : 420.5); (positive mode):m/z153.4 (M+H - ) (calcd. for M+H - : 153.14). 1 HNMR (300 MHz,DMSO-d6):δ 1.04 (m,2H),1.26 (m,2H), 1.61 (m,9H),1.77 (m,4H),2.13 (m,1H),2.66 (d,2H),3.24-3.55 (m, 6H),3.62 (m,2H),7.64 (t,1H),7.83 (t,1H),7.96 (t,2H),8.45 (d,1H), 8.81 (d,1H). Anal. Calcd. for C32H35N3O5S: C,66.99; H,6.15; N,7.32; S,5.59; Found: C,65.67; H,6.28; N,7.22; S,5.47.
Photopolymerization: PIs (3×10 -5 mol) composed of thioxanthen-DBU,BPO,or ITX were dissolved in DMSO (0.5 mL) under ultrasonication,and then TMPTA (1 g) was added to this solution. The mixture was injected into a mold,and irradiated with an optical cable-directed UV lamp in the 200-400 nm range (RW-UVA-F200U,Runwing Co.,China). The light intensity at the surface level of the cured samples was measured to be 20 mW/cm 2 by a UV-radiometer (type UV-A,Photoelectric Instrument Factory, Beijing Normal University).
Photo-generated DBU detected by ESI-MS: A thioxanthen-DBU solution in DMSO (0.01 mmol/L) was irradiated for 30 min,and then isometric water was added,nonphotolytic thioxanthen-DBU was filtered off. ESI-MS spectrum of the filtrated solution was then measured. 3. Results and discussion
Synthesis of the photocaged base thioxanthen-DBU was achieved according to the procedures given in Scheme 1. Firstly, the NO2group in 3-nitrophthalimide was displaced by salicylic acid sodium salt (f),followed by an intramolecular acylation of the obtained intermediate ein polyphosphoric acid (PPA). Secondly, the hydrolysis of d in alkaline aqueous solution gave the dicarboxylic acid c,which reacted with acetic anhydride to produce the 9-oxo-9H-thioxanthene-3,4-anhydride b. Finally, thioxanthone-based N-phthalimidoamino acid ammonium salt (thioxanthen-DBU) was synthesized from trans-4-(aminomethyl)-cyclohexanecarboxylic acid and the anhydride b,followed by a salt formation reaction with 1,8-diazabicyclo[5.4.0] undec-7-ene (DBU). The obtained thioxanthen-DBU was characterized by 1 H NMR,elemental analysis,and ESI-MS spectra.

|
Download:
|
| Scheme 1.Synthesis route for thioxanthen-DBU. | |
TX derivatives have extended absorption up to 420 nm depending upon the type of substitutions [22]. Fig. 1 demonstrates the absorption spectrum of thioxanthen-DBU. It exhibits good absorption characteristics with a maximum at 285 nm and 418 nm and surprisingly a tail over 480 nm. A clear red shift was observed compared with TX due to the amide substituents on the TX skeleton. The n→p* absorption band appears in the visible region and high molar absorptivity may allow this photocaged base to be used in day light polymerization applications (instead of medium pressure mercury lamps). Furthermore,photogenerated protonated DBU (m/z153.4) can be detected by ESI-MS spectra after 30 min of irradiation (Fig. 2),which was the direct evidence for the generation of DBU from irradiated thioxanthen-DBU.

|
Download:
|
| Fig. 1.UV-vis absorption spectra of thioxanthen-DBU (1×10 -5 mol/L) in DMSO. | |

|
Download:
|
| Fig. 2.Positive ion ESI-MS spectra of irradiated thioxanthen-DBU in DMSO (0.01 mol/L). | |
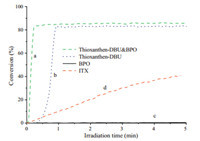
The catalytic behaviors of the photocaged redox initiator combination (thioxanthen-DBU&BPO),and its parent compounds, thioxanthen-DBU and BPO,were compared using real-time FTIR spectroscopy at room temperature (Fig. 3). As depicted in Table 1, control experiments (entries 1 and 2) indicate that both the photocaged base and photoirradiation are essential to trigger this redox photopolymerization. The photopolymerization of TMPTA in the presence of thioxanthen-DBU was fast (Fig. 3,curve b),80% final conversion was achieved in 1 min. As described in the previous work [20, 24],planarN-aromatic maleimides do not generate radicals and initiate photopolymerization. Hence,the photopolymerization initiated by thioxanthen-DBU alone should be attributed to the structure of TX. On the other hand, BPO alone cannot initiate a clear polymerization (Fig. 3,curve c). Interestingly,the thioxanthen-DBU&BPO combination can invoke the newborn amine-mediated redox radical polymerization (Fig. 3,curve a),and 82.5% yield was obtained in 0.22 min. Compared to thioxanthen-DBU,the induction period (Tstart )of the two-component redox system was obviously shortened from 0.3 min to 0.03 min and the maximum initiation rate (Rpmax) dramatically increased from 378 min -1 to 580 min -1 ,indicating this two-component catalytic system proceeded rapidly and efficiently. Due to the one-component structure of the thioxanthen-DBU,both the photosensitizer and the hydrogen bond donor are combined together therefore this type of PI does not require an additional co-initiator. Hence,compared to the conventional PI ITX (Fig. 3,curve d),the superiority of thioxanthen-DBU can be clearly observed.
|
Download:
|
| Fig. 3.Conversionvstime for the photopolymerization of TMPTA in the presence of: (a) thioxanthen-DBU&BPO,(b) thioxanthen-DBU,(c) BPO,and (d) ITX. | |
| Table 1 Photopolymerizations of TMPTA initiated by BPO,thioxanthen-DBU,and thioxanthen-DBU&BPO combination. |
The involvement of a decarboxylation process during the photolysis ofN-phthalimidoamino acid derivative was described in the literature [24, 25]. Furthermore,as proposed in the literatures [26, 27, 28],this kind of acetic acid-based thioxanthone derivative that self-quenching from the triplet state could lead to the formation of the initiating radicals during the decarboxylation process. After extrusion of CO2,the corresponding methyl radical is possibly responsible for the initiation of the polymerization of TMPTA. Moreover,photogenerated DBU can transiently activate the oxidation-reduction polymerization in conjunction with the oxidant BPO. Taking the foregoing points into consideration,the principal photoreaction pathways of thioxanthen-DBU and BPO are laid out in Scheme 2.
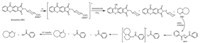
|
Download:
|
| Scheme 2.Proposed mechanism of photoinduced radical generation. | |
In the paper,a photocaged base (thioxanthen-DBU) using Thioxanthen as the peripheral chromophore ofN-phthaloyltranexamic acid ammonium salt is proposed. In combination with a benzoyl peroxide initiator,its ability to initiate free radical photopolymerization of TMPTA was demonstrated and compared with that of the parent thioxanthen-DBU,BPO,and conventional PI ITX. It is found that thioxanthen-DBU&BPO is a highly effective photoinitiator combination. The mechanism of the radical formation to initiate the redox photopolymerization is proposed. This photodecarboxylation reaction is of particular interest as it is can facilitate the design of new photocaged bases with very promising properties. Acknowledgments This research was financially supported by National Natural Science Foundation of China (No. 20974127,21374135),China Postdoctoral Science Foundation (No. 2013M542178),the Open Foundation of the State Key Laboratory of Pulp and Paper Engineering in South China University of Technology (No. C713043z),and the Fundamental Research Funds for the Central Universities (No. 2013ZB0025).
| [1] | Y. Yagci, S. Jockusch, N.J. Turro, Photoinitiated polymerization: advances, challenges, and opportunities, Macromolecules 43 (2010) 6245-6260. |
| [2] | L. Gonsalvi, M. Peruzzini, Novel synthetic pathways for bis (acyl) phosphine oxide photoinitiators, Angew. Chem. Int. Ed. 51 (2012) 7895-7897. |
| [3] | J.V. Crivello, E. Reichmanis, Photopolymer materials and processes for advanced technologies, Chem. Mater. 26 (2014) 533-548. |
| [4] | Y.L. Xu, H.J. Xu, X.S. Jiang, J. Yin, Versatile functionalization of the micropatterned hydrogel of hyperbranched poly(ether amine) based on "thiol-yne" chemistry, Adv. Funct. Mater. 24 (2014) 1679-1686. |
| [5] | M. Tehfe, F.E.D.E. Dumur, P. Xiao, et al., Chalcone derivatives as highly versatile photoinitiators for radical, cationic, thiol-ene and IPN polymerization reactions upon exposure to visible light, Polym. Chem. 5 (2014) 382-390. |
| [6] | J.L. Yang, S.Q. Shi, F. Xu, J. Nie, Synthesis and photopolymerization kinetics of benzophenone sesamol one-component photoinitiator, Photochem. Photobiol. Sci. 12 (2013) 323-329. |
| [7] | H. Tar, D. Sevinc Esen, M. Aydin, et al., Panchromatic type Ⅱ photoinitiator for free radical polymerization based on thioxanthone derivative, Macromolecules 46 (2013) 3266-3272. |
| [8] | H. Chen, Z.L. Zou, S.L. Tan, et al., Efficient synthesis of water-soluble calix[4]arenes via thiol-ene "click" chemistry, Chin. Chem. Lett. 24 (2013) 367-369. |
| [9] | Y.Y. Cui, Y.E. Ren, X.X. Liu, Synthesis of methyl methacrylate star-branched polymer with divinylbenzene as a linking agent via controlled/living photopolymerization, Chin. Chem. Lett. 23 (2012) 985-988. |
| [10] | M.H. He, X. Huang, Y.G. Huang, Z.H. Zeng, J.W. Yang, Photoinduced redox initiation for fast polymerization of acrylaytes based on latent superbase and peroxides, Polymer 53 (2012) 3172-3177. |
| [11] | M.H. He, X. Huang, Z.H. Zeng, J.W. Yang, Photo-triggered redox frontal polymerization: a new tool for synthesizing thermally sensitive materials, J. Polym. Sci. A: Polym. Chem. 51 (2013) 4515-4521. |
| [12] | M.H. He, X. Huang, Z.H. Zeng, J.W. Yang, Phototriggered base proliferation: a highly efficient domino reaction for creating functionally photo-screened materials, Macromolecules 46 (2013) 6402-6407. |
| [13] | M.H. He, S. Jiang, R.X. Xu, et al., Domino free radical photopolymerization based on phototriggered base proliferation reaction and redox initiation, J. Polym. Sci. A: Polym. Chem. 52 (2014) 1560-1569. |
| [14] | G. Yilmaz, B. Aydogan, G. Temel, et al., Thioxanthone-fluorenes as visible light photoinitiators for free radical polymerization, Macromolecules 43 (2010) 4520- 4526. |
| [15] | D.K. Balta, G. Temel, G. Goksu, et al., Thioxanthone-diphenyl anthracene: visible light photoinitiator, Macromolecules 45 (2011) 119-125. |
| [16] | D. Tunc, Y. Yagci, Thioxanthone-ethylcarbazole as a soluble visible light photoinitiator for free radical and free radical promoted cationic polymerizations, Polym. Chem. 2 (2011) 2557-2563. |
| [17] | G. Yilmaz, S. Beyazit, Y. Yagci, Visible light induced free radical promoted cationic polymerization using thioxanthone derivatives, J. Polym. Sci. A: Polym. Chem. 49 (2011) 1591-1596. |
| [18] | M.A. Tehfe, F. Dumur, B. Graff, et al., Design of new Type I and Type Ⅱ photoinitiators possessing highly coupled pyrene-ketone moieties, Polym. Chem. 4 (2013) 2313-2324. |
| [19] | H.Y. Wang, J. Wei, X.S. Jiang, J. Yin, Highly efficient, polymerizable, sulfur-containing photoinitiator comprising a structure of planar N-phenylmaleimide and benzophenone for photopolymerization, J. Polym. Sci. A: Polym. Chem. 44 (2006) 3738-3750. |
| [20] | H. Wang, J. Wei, X. Jiang, et al., Novel chemical-bonded polymerizable sulfurcontaining photoinitiators comprising the structure of planar N-phenylmaleimide and benzophenone for photopolymerization, Polymer 47 (2006) 4967- 4975. |
| [21] | S.K. Dogruyol, Z. Dogruyol, N. Arsu, A thioxanthone-based visible photoinitiator, J. Polym. Sci. A: Polym. Chem. 49 (2011) 4037-4043. |
| [22] | W. Fischer, Aromatic nucleophilic substitution. Part 3. Preparation of novel 9-oxo- 9H-thioxanthene-and 9-oxo-9H-xanthenedicarboximides and-dicarboxylates, Helv. Chim. Acta 74 (1991) 1119-1126. |
| [23] | C.W. Miller, E.S. Jö nsson, C.E. Hoyle, K. Viswanathan, E.J. Valente, Evaluation of Naromatic maleimides as free radical photoinitiators: a photophysical and photopolymerization characterization, J. Phys. Chem. A 105 (2001) 2707-2717. |
| [24] | Y. Takahashi, T. Miyashi, U.C. Yoon, et al., Mechanistic studies of the azomethine ylide-forming photoreactions of N-(silylmethyl) phthalimides and N-phthaloylglycine, J. Am. Chem. Soc. 121 (1999) 3926-3932. |
| [25] | H.G.O. Rner, A.G. Griesbeck, T. Heinrich, et al., Time-resolved spectroscopy of sulfur-and carboxy-substituted N-alkylphthalimides, Chem. Eur. J. 7 (2001) 1530-1538. |
| [26] | M. Aydin, N. Arsu, Y. Yagci, S. Jockusch, N.J. Turro, Mechanistic study of photoinitiated free radical polymerization using thioxanthone thioacetic acid as onecomponent type Ⅱ photoinitiator, Macromolecules 38 (2005) 4133-4138. |
| [27] | M. Aydin, N. Arsu, Y. Yagci, One-component bimolecular photoinitiating systems, 2, Macromol. Rapid Commun. 24 (2003) 718-723. |
| [28] | D.K. Balta, G. Temel, M. Aydin, N. Arsu, Thioxanthone based water-soluble photoinitiators for acrylamide photopolymerization, Eur. Polym. J. 46 (2010) 1374-1379. |





