Bisphenol-A [2,2-bis(4-hydroxyphenyl)propane; BPA] is mainly used for the production of polycarbonate and epoxy resins. It is commonly prepared by the condensation of phenol and acetone and the catalyst plays a very important role in this reaction. Till now,some novel catalysts have increasingly drawn attention,such as layered silicate [1],mesoporous MCM silica [2, 3, 4],heteropoly acids [5, 6, 7],and zeolites [8]. These catalysts, although promising,still require considerable R&D efforts prior to the commercial scale applications. Since 1960 when U.C. Corporation [9] first brought the cation exchange resins into industrialized application for the BPA production,ion exchange resins have been widely used in industry due to their high selectivity,easy separation and minimal equipment corrosion. However,poor thermal stability and low acidity are their intrinsic drawbacks.Atthesametime,partialexchange of H+ between cationic impurities from reactants and the resin accelerates the deactivation of the catalyst.
To overcome the shortcomings of ion exchange resins, numerous efforts have been made,including loading thiol groups and/or amine groups by means of reduction [10],esterification [11],neutralization [12] or ion exchange method [13]. Takahim and Toshitaka [14] successfully synthesized a modified catalyst with mercapto alkyl amine,which showed a great improvement in the condensation of phenol and acetone. Carvill et al. [15] discovered that 4-(2-mercaptoethyl)-pyridine was a good modified reagent. Terajima et al.[16] invented a novel thiol-modified resin catalyst with a phosphate structure,and it proved to possess very high activity. These methods have obviously achieved great improvements,but they are also greatly limited by the complexity in synthesis and easy deactivation.
Ion exchange resins have been used in other reactions,such as esterification [17],transesterification [18],oligomerization [19]. Low acid strength is also one of their main drawbacks affecting the reaction efficiency. Some researchers attempted to solve this issue by introducing Lewis acids into the resins [20, 21, 22, 23]. Magnotta and Gates [20] reported that the acidic property of the complex formed by AlCl3-sulfonic acid can be similar to that of the superacid solution of SbF5+ FSO3H. Shi et al.[23] showed that the efficiency of acid-catalyzed transesterification and esterification reactions depend on the subtle balance between Lewis and Brønsted acidities. It has been proved that the coordination of a Lewis acid with a Brønsted acid can increase its original acidity [24]. Therefore,the design of dual acid catalysts based on resins can be advantageous in the BPA production. In this study,ZnCl2 acting as a Lewis acid was added into cationic ion exchange resins to fabricate a more efficient catalyst. To the best of our knowledge, this is the first resin catalyst for producing BPA that contains both a Brønsted acid and a Lewis acid. 2. Experimental 2.1. Catalyst preparation
Phenol (99.8%),acetone (≥99.9%) and ethanol (≥99.5%) were purchased from Tianjin Reagent Company (China). AlCl3(≥97.0%), FeCl3 (≥99.0%),SnCl2 (≥98.0%),ZnCl2 (≥98.0%) were purchased from Tianjin Guangfu Company (China).
A strong acidic ion-exchange resin (Amberlyst-15,Rohm and Haas Company) was washed by ethanol and deionized water with the speed of 15 cm/min until the effluent was colorless,and was subsequently dried in an oven for 24 h at 70℃. Certain amount of ZnCl2was dissolved in ethanol to produce different concentrations (w/w); then 5 g of treated ion exchange resin was added into the ZnCl2 solution (100 mL) and kept for 6 h at room temperature. Finally the modified catalyst was washed with deionized water until no Cl- exists in the solution,and then was dried in a vacuum oven for 48 h at 70℃.
The preparation of AlCl3,FeCl3 and SnCl2 modified catalysts employed the same procedure as that of ZnCl2 modified one mentioned above. 2.2. Catalyst characterization
The crystal morphology was observed on a Hitachi S-4800 SEM at 5.0 kV. FT-IR spectra were recorded in a Nicolet NEXUS spectrophotometer using KBr pellets in the 4000-400 cm-1 region.IR spectrum of pyridine adsorption was also used to obtain the type of acid sites ranging from 1700 cm-1 to 1300 cm-1; the samples were pre-treated under vacuum at 70℃ for 2 h prior to the adsorption of pyridine at room temperature for 48 h and subsequently desorbed at 100℃ for 1 h to physically remove adsorbed pyridine. Thermo gravimetric analysis curves were collected using a Perkin Elmer Diamond instrument with a heating rate of 10℃/min from 30 to 800℃,and the flow rate of N2 is 40 mL/min.
The acid strength of the catalysts was determined by the Hammett indicator method.
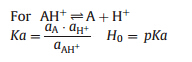
The synthesis of BPA was carried out in the liquid phase under atmospheric pressure in a 250 mL three-neck round-bottom flask equipped with a condenser,a magnetic stirrer and a thermometer. Phenol (13.6 g) and catalyst (2.0 g) were added into the reactor, and the reactants were heated at 70℃ for 5 h with a stirring rate of 800 revolutions per minute (rpm),then acetone (1.7 g) was added. After 150 min,the reaction mixture was cooled to 30℃, then the catalyst was separated from the reaction solution. Reaction products were analyzed by an HPLC (Agilient 810) and methanol/water (60:40,v/v) was used as the mobile phase with the flow rate of 1.0 mL/min.
Catalytic results were recorded as conversion (CAcetone,wt%, based on acetone) and selectivity (SBPA,wt%),which were calculated by HPLC analysis. These parameters are defined as
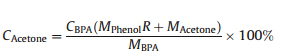
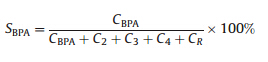
Fig. 1 illustrates the catalytic activity affected by the concentration of ZnCl2 (Fig. 1a) and exchange time (Fig. 1b), respectively. Catalytic activity was enhanced greatly with increased ZnCl2concentration before 2.0% (Fig. 1a). The coordination of Zn2+ with a sulfonic acid group formed a stable Brønsted-Lewis acid site. But we should note that an excessive amount of loaded ZnCl2 could occupy active sites and decrease effective catalytic surface area. Consequently,the optimal concentration of ZnCl2is 2.0%. The same tendency was observed for the exchange time (Fig. 1b). When the exchange time exceeded 6 h the conversion and selectivity changed little,for Amberlyst-15 ion exchange resin has a fixed exchange volume of 4.7 mmol/g [25].

|
Download:
|
| Fig. 1.Effects of preparation conditions on catalytic activity of ZnCl2-modified ion exchange resin catalyst for phenol and acetone condensation. (a) ZnCl2concentration; (b) exchange time. Reaction conditions: phenol 13.6 g,acetone 1.7 g,catalyst 2.0 g,70℃,150 min,stirring rate 800 rpm. | |
The SEM images were obtained to show surface morphologies of the catalysts before (Fig. 2a and c) and after modification (Fig. 2b and d). Fig. 2a shows that there are many cracks on the surface of the unmodified catalyst due to the introduction of the sulfonic acid groups during the sulfonation process [26]. After being modified,it had fewer or no cracks (Fig. 2b). The pore diameter became much smaller than the unmodified ones (Fig. 2c and d). This can be attributed to the fact that ZnCl2and sulfonic acid groups can form a new network structure.

|
Download:
|
| Fig. 2.SEM images of (a,c) unmodified and (b,d) ZnCl2-modified catalysts. | |
The IR spectra of pyridine adsorbed on the samples are shown in Fig. 3a. The intense peak at 1550 cm-1 was attributed to the Brønsted acid site. After modification two new peaks appeared at 1486 cm-1 and 1435 cm-1 ,which corresponded to the Brønsted- Lewis site and the Lewis acid site,respectively [27]. The measurement result indicates that the Lewis acid was successfully introduced to the resin catalyst by partially exchanging H+ with Zn2+.

|
Download:
|
| Fig. 3.(a) IR spectra of pyridine adsorbed on unmodified and ZnCl2-modified catalysts. B: Brønsted acid site,L: Lewis acid site. (b) FT-IR spectra of unmodified and ZnCl2-modified catalysts. | |
The FT-IR spectrum indicates that the basic skeleton of the resin did not changed except the modified one contained a new absorption peak at 2358 cm-1 shown in Fig. 4b,which could be due to the asymmetric stretching vibration of S=O in -SO3H [28]. After the resin modified with ZnCl2,Zn2+ interacted with the lone pair electron of the O to form ap-dcoordinate bond,which could make the two S=O asymmetric. This bond can exist either in one molecule or between two molecules,thus forming a peripheral shielding that stabilizes the sulfonic acid groups. Moreover,thep electron on the S can be transferred to the empty orbitals of Zn2+. The positive charge can be delocalized in the sulfonic acid group [27] thereby greatly improved the stability of the ion exchange resin.

|
Download:
|
| Fig. 4.TGA patterns of (a) unmodified and (b) ZnCl2-modified catalysts. | |
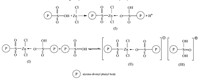
As shown in Scheme 1,ZnCl2can coordinate with the sulfonic
acid group to form structure (Ⅰ),which is unstable and can
reversibly form structure (Ⅱ) and structure (Ⅲ) [28]. It can be seen
that structure (Ⅲ) is a strong proton-donor that possesses a high
acid strength. The Hammett indicator method was used to
measure the acid strength of the catalysts. The ZnCl2 modified
catalyst (-8.2 Fig. 4 shows the TGA patterns of unmodified catalyst and 2.0%
ZnCl2modified one. Three kinds of weight loss exist in this process
for the unmodified catalyst (Fig. 4a,curve DTG),and it is generally
considered that the first peak (80℃) is produced by the removal of
solvent,the second (300℃) corresponds to the removal of the
sulfonic acid group and the last (520℃) is responsible for the
resolving of the styrene-divinyl phenyl body [29].
After being modified the second peak split into two peaks
(Fig. 4b,curve DTG). According to the analysis of the FT-IR spectra
above,the coordination of Zn2+
with the sulfonic acid group
changed the structure of the catalyst,leading to a two-step process
for the sulfonation removal (the removal of single sulfonic acid
groups and the removal of sulfonic acid groups coordinating
with metal ions). Thus the sulfonic acid group was protected by
coordination and still functioned well at a relatively high
temperature. Accordingly,the thermal stability of the catalyst
has been significantly improved.
AlCl3,FeCl3and SnCl2modified catalysts were also prepared
as references,and the preparation conditions were the same as
that of ZnCl2modified one. The electronegativities of Al3+
,Fe3+,Zn2+ and Sn2+
are 26.72,15.38,10.38 and 8.12,respectively [30],
and the electron acceptability of these cations is in parallel to the
electronegativity. So the sequence of these Lewis acids’ strength is
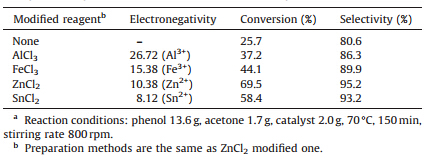
AlCl3>FeCl3>ZnCl2>SnCl2. Table 1 demonstrates the catalytic
activity of different Lewis acid modified catalysts. Moderate
strength Lewis acid modified catalyst was found to produce the
best catalytic activity. A certain strength of Lewis acid is obviously
needed to form stable Brønsted-Lewis active sites to increase the
proton-donating ability,thus making acetone more easily protonated to form a stable carbenium ion to initiate the reaction
(Scheme 2,step I). However,it is important to note here that a
stronger Lewis acid do not necessarily results in a better catalytic
activity. If a metal cation is an overly strong Lewis acid,it is
speculated to be prone to coordinate with acetone,which prohibits
acetone to undergo further reaction. Therefore,metal cations with
intermediate Lewis acidity provide the highest catalytic activity.
We have successfully fabricated a ZnCl2-modified ion exchange
resin that possesses excellent catalytic activity for the synthesis of
bisphenol-A. The final conversion of acetone and selectivity of BPA
are 69.5% and 95.7%,respectively. The increased activity and
stability after adding Lewis acid have been preliminarily explained
by analyzing the IR spectra and the rein structure coordinating
with ZnCl2. The TGA patterns also indicated that the thermal
stability of the resin catalyst was dramatically increased after
modification. Compared with the widely used thiol-modified ion
exchange resin,the ZnCl2-modified catalyst is easier to prepare and
less susceptible to oxidation. The synergistic effect of Brønsted and
Lewis acids not only extends the use of resin catalysts in industry,
but also provides a guideline for the design of new catalysts.
Scheme 1.Coordination reaction between sulfonic acid group and ZnCl2.
![]()
Table 1
Catalytic activity of different Lewis acid modified catalysts.a

Scheme 2.Reaction mechanism of bisphenol-A synthesis on acidic catalyst.
| [1] | Y. Ide, N. Kagawa, M. Itakura, et al., Effective and selective bisphenol A synthesis on a layered silicate with spatially arranged sulfonic acid, ACS Appl. Mater. Int. 4 (2012) 2186-2191. |
| [2] | D. Das, J.F. Lee, S. Cheng, Sulfonic acid functionalized mesoporous MCM-41 silica as a convenient catalyst for bisphenol-A synthesis, Chem. Commun. 6 (2001) 2178-2179. |
| [3] | D. Das, Selective synthesis of bisphenol-A over mesoporous MCM silica catalysts functionalized with sulfonic acid groups, J. Catal. 223 (2004) 152-160. |
| [4] | W. Kaleta, K. Nowiń ska, Immobilisation of heteropoly anions in Si-MCM-41 channels by means of chemical bonding to aminosilane groups, Chem. Commun. 6 (2001) 535-536. |
| [5] | K. Nowiń ska, W. Kaleta, Synthesis of bisphenol-A over heteropoly compounds encapsulated into mesoporous molecular sieves, Appl. Catal. A 203 (2000) 91- 100. |
| [6] | K. Shimizu, S. Kontani, S. Yamada, et al., Design of active centers for bisphenol-A synthesis by organic-inorganic dual modification of heteropolyacid, Appl. Catal. A 380 (2010) 33-39. |
| [7] | G.D. Yadav, N. Kirthivasan, Synthesis of bisphenol-A: comparison of efficacy of ion exchange resin catalysts vis-à -vis heteropolyacid supported on clay and kinetic modelling, Appl. Catal. A 154 (1997) 29-53. |
| [8] | A. Singh, Preparation of bisphenol-A over zeolite catalysts, Catal. Lett. 16 (1992) 431-435. |
| [9] | B.S. Zheng, Present station of BPA at home and abroad and suggestions on development of China's BPA, Fine Spec. Chem. 12 (2001) 7-10. |
| [10] | R.B. Wagner, A-preparation using ion exchange resin containing mercatpo and sulfic acid groups, US Patent 3 172 916 (1965). |
| [11] | N.A. Francis, B.C. Louis, Cationic exchange polymeric resin, US Patent 3 153 001 (1964). |
| [12] | B. Wang, H. Sun, J. Zhu, L. Wang, S. Chen, Bis(2-mercapto-ethyl) amine modification of macroporous sulfonic tesin catalyst in bisphenol-A synthesis, AIChE J. 59 (2013) 3816-3823. |
| [13] | B.B. Gammill, G.R. Ladewig, G.E. Ham, Ion exchange catalyst for the preparation of bisphenols, US Patent 3 760 006 (1973). |
| [14] | S. Takahim, S. Toshitaka, Ion exchange resin, JP Patent 8 089 819 (1996). |
| [15] | B. Carvill, K. Glasgow, G. Kishan, Process for the synthesis of bisphenol, US Patent 0 116 751A (2004). |
| [16] | T. Terajima, T. Takai, H. Nakamura, Modified ion exchange resin and process for producing bisphenols, US Patent 0 224 315A1 (2011). |
| [17] | B. Schmid, M. Dö ker, J. Gmehling, Esterification of ethylene glycol with acetic acid catalyzed by amberlyst 36, Indust. Eng. Chem. Res. 47 (2008) 698-703. |
| [18] | M. Kim, S. Salley, K. Ng, Transesterification of glycerides using a heterogeneous resin catalyst combined with a homogeneous catalyst, Energy Fuels 22 (2008) 3594-3599. |
| [19] | M. Granollers, J. Izquierdo, F. Cunill, Effect of macroreticular acidic ion-exchange resins on 2-methyl-1-butene and 2-methyl-2-butene mixture oligomerization, Appl. Catal. A 435-436 (2012) 163-171. |
| [20] | V. Magnotta, B. Gates, Superacid polymers: synthesis and analysis of AlCl3- sulfonic acid resin complexes, J. Polym. Sci. 15 (1977) 1341-1347. |
| [21] | J. Penzien, C. Haeßner, A. Jentys, et al., Heterogeneous catalysts for hydroamination reactions: structure-activity relationship, J. Catal. 221 (2004) 302-312. |
| [22] | K. Shimizu, H. Furukawa, N. Kobayashi, Y. Itaya, A. Satsuma, Effects of Brønsted and Lewis acidities on activity and selectivity of heteropolyacid-based catalysts for hydrolysis of cellobiose and cellulose, Green Chem. 11 (2009) 1627-1632. |
| [23] | W. Shi, J. Zhao, X. Yuan, et al., Effects of Brønsted and Lewis acidities on catalytic activity of heteropolyacids in transesterification and esterification reactions, Chem. Eng. Technol. 35 (2012) 347-352. |
| [24] | H. Yamamoto, From designer Lewis acid to designer Brønsted acid towards more reactive and selective acid catalysis, Proc. Jpn. Acad. Ser. B: Phys. Biol. Sci. 84 (2008) 134-146. |
| [25] | M.L. Honkela, A. Root, M. Lindblad, A.O.I. Krause, Comparison of ion-exchange resin catalysts in the dimerisation of isobutene, Appl. Catal. A 295 (2005) 216- 223. |
| [26] | F. Coutinho, R.R. Souza, A.S. Gomes, Synthesis, characterization and evaluation of sulfonic resins as catalysts, Eur. Polym. J. 40 (2004) 1525-1532. |
| [27] | L.X. Mao, The preparation of cyclohexene by dehydration of cyclohexanol on Ti4+ modificatory cation exchange resin catalyst, Ion Exch. Adsor. 20 (2004) 562-568. |
| [28] | F. Zhang, X. Jiang, J. Hong, H. Lou, X. Zheng, Preparation of ZnCl2-modified ion exchange resin and its catalytic activity for esterification of ethanol and acetic acid under microwave, Chin. J. Catal. 31 (2010) 666-670. |
| [29] | N. Bothe, F. Dö scher, J. Klein, H. Widdecke, Thermal stability of sulphonated styrene-divinylbenzene resins, Polymer 20 (1979) 850-854. |
| [30] | Y. Feng, F. Zhang, Ionic electronegativity, J. Dallan Inst. Light Ind. 17 (1998) 70-76. |