b Key Laboratory of Special Functional Aggregated Materials, Ministry of Education, School of Chemistry and Chemical Engineering, Shandong University, Ji'nan 250100, China
Dendrimers are highly branched macromolecules with welldefined architectures and surface functionality that are obtained from iterative stepwise procedures. They have been one of the most extensively studied materials during the last decade because of their controllable nanosize,unique topological structure,and versatile physicochemical properties,and were widely used in the fields of catalysis,medicinal chemistry,and nanotechnologies[1, 2, 3]. In recent years,there has been a rapid growth in the discovery and application of new dendrimers. Carbosilane dendrimers are among the most widely used because of their high flexibility, catalytic inertness,and accessibility [4]. These dendrimers typically possess low glass transition temperatures and relatively low viscosities. They are kinetically and thermodynamically stable owing to the low polarity of the Si-C bond and its high bond strength (306 kJ/mol),which is very similar to that of C-C bond (345 kJ/mol) [5]. In addition,the availability and versatility of many synthetic reactions in organosilicon chemistry enable the facile synthesis of these dendrimers. As a result,the synthesis,characterization,and application of different types of carbosilane dendrimers have attracted wide attention [6, 7, 8].
Carbosilane dendrimers are generally synthesized by the divergent method. They are built stepwise from the central core that possesses alkenyl groups through the reiteration of sequential hydrosilylations with chlorosilanes and alkenylations with Grignard reagents,which allow the construction of dendrimers with various generations [9]. The synthetic route of carbosilane dendrimers offers high flexibility and versatility as the hydrosilylation and alkenylations reagents can be varied accordingly. Especially,the hydrosilylation reaction,an addition of a hydrosilane unit (Si-H) to a double bond,has been proved to be high yielding and selective and represents a powerful tool for the rapid and efficient synthesis of carbonsilane dendrimers [10]. In our previous studies,a series of acetyl,N,N-dimethylaniline,and platinum-capped carbosilane dendrimers were synthesized [11, 12, 13]. However,the main drawback of these dendrimers is that such functional groups exhibit relative low reactivity,which may limit further functionalization and application of the carbosilane dendrimers. The synthesis of carbosilane dendrimers with reactive functional groups at periphery is still a great challenge.
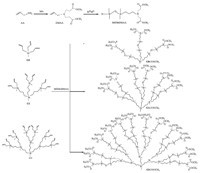
In the present study,a series of ester-capped carbosilane dendrimers were synthesized via a hybrid divergent-convergent method and their structures were confirmed by FTIR,1H NMR,and HRMS analyses. The peripheral reactive ester functional groups can be further functionalized using appropriate synthetic procedures to expand the application of carbosilane dendrimers. 2. Experimental Fourier transform infrared spectra (FTIR) were recorded on a Bruker Tensor 27 spectrophotometer (Bruker,Switzerland) in the range of 400-4000 cm-1 with a resolution of 4 cm-1 by accumulating 32 scans. The data were treated with an OPUS spectroscopy software of version 6.1H NMR was measured in chloroform on a Bruker AVANCE-400 NMR Spectrometer,and chemical shifts were recorded in parts per million (ppm) without an internal reference. Molecular weights were determined by an Agilent Technologies 6510 Q-TOF LC-MS (HRMS). Methyl acrylate (MA),ethylenediamine (EDA),allylamine (AA),1,1,3,3-tetramethyldisiloxane (MHMH) and tetrahydrofuran (THF) were redistilled just before use. All other reagents used were of analyticalreagent grade. The starting materials carbosilane dendrimers G0, G1,and G2 were prepared according to the method described in Ref. [13]. DMAA was prepared by a Michael addition reaction ofMA with AA according to the procedure described in our previous work [14]. The synthetic routes of ester-capped carbosilane dendrimers are illustrated in Scheme 1.
|
Download:
|
| Scheme 1.The hybrid divergent-convergent synthesis routes of ester-capped carbosilane dendrimers. | |
MHM-DMAA was prepared according to a similar method described in Ref. [14]. A suspension of 2.68 g (0.020 mol) of MHMH and three drops of the Karstedt catalyst were added into the flask and the mixture was heated slowly at 70 ℃ under argon atmosphere. Then 1.15 g (0.0050 mol) of DMAA was added drop wise with stirring and the reaction mixture was allowed to stir for 12 h. After the reaction completed,the excess MHMH was removed under reduced pressure and 1.70 g (0.0047 mol) of MHM-DMAA was obtained. Yield: 94.10%.
FTIR (KBr,cm-1): υ 2969,2863 (CH3,CH2),2110 (SiH),1738 (COOCH3),1251 (Si-CH3),1041 (Si-O-Si); 1H NMR (CDCl3): δ-0.07 to 0.02 (m,12H,SiCH3),0.30-0.35 (t,2H,SiCH2CH2CH2N),1.29 (m,2H,SiCH2CH2CH2N),2.29-2.33 (m,6H,SiCH2CH2 CH2NCH2),2.62-2.65 (m,4H,NCH2CH2CO),3.52 (s,6H,OCH3),4.52 (s,H,SiH). 2.2. Synthesis of G0-COOCH3
G0-COOCH3 was synthesized via the hydrosilylation reaction between G0 and MHM-DMAA similar to the procedures described elsewhere [11]. A sample of 0.17 g (0.0010 mol) G0,1.34 g (0.0036 mol) MHM-DMAA,and three drops of the Karstedt catalyst were suspended in 30 mL THF under argon atmosphere. The mixture was stirred at 65 8C for 12 h,then the solvent and remaining MHM-DMAA were removed under reduced pressure, and 0.98 g (0.78 mmol) of G0-COOCH3 was obtained. Yield: 78.0%.
FTIR (KBr,cm-1): υ 2952,2873 (CH3,CH2),1737 (COOCH3), 1251 (Si-CH3),1041 (Si-O-Si); 1H NMR (CDCl3): δ 0.00-0.10 (m,39H,SiCH3),0.38-0.48 (m,12H,SiCH2CH2CH2Si),0.50-0.59 (m,6H,SiCH2CH2CH2N),1.24-1.45 (m,12H,SiCH2CH2CH2Si, SiCH2CH2CH2N),2.36-2.53 (m,18H,SiCH2CH2CH2NCH2),2.73-2.91(t,12H,NCH2CH2CO),3.65 (s,18H,OCH3); HRMS: m/z 1256.7007 [MH]+ (calcd. 1255.6864). 2.3. Synthesis of G1-COOCH3
Under argon atmosphere,a suspension of 0.24 g (0.0004 mol) G1,1.34 g (0.0036 mol) MHM-DMAA,and three drops of the Karstedt catalyst in 30 mL THF was stirred at 65 ℃ for 16 h. After the reaction is complete,the purification procedures similar to those of G0-COOCH3 were used to produce 0.39 g (0.143 mmol) G1-COOCH3. Yield: 72.1%.
FTIR (KBr,cm-1): υ 2953,2873 (CH3,CH2),1738 (COOCH3), 1251 (Si-CH3),1040 (Si-O-Si); 1H NMR (CDCl3): δ 0.02-0.10 (m,84H,SiCH3),0.38-0.45 (m,24H,SiCH2CH2CH2Si),0.51-0.59 (m,24H,SiCH2CH2CH2N),1.25-1.35 (m,18H,SiCH2CH2CH2Si), 1.37-1.45 (m,12H,SiCH2CH2CH2N),2.40-2.46 (m,36H, SiCH2CH2CH2NCH2),2.75-2.79 (t,24H,NCH2CH2CO),3.66 (s, 36H,OCH3); HRMS (FAB): m/z 2724.5228 [MH]+ (calcd.2723.5150). 2.4. Synthesis of G2-COOCH3
A solution of 0.65 g (0.0005 mol) G2,3.63 g (0.01 mol) MHMDMAA, five drops of the Karstedt catalyst in 100 mL THF was stirred under argon atmosphere at 65 ℃ for 24 h. After the reaction completed,the purification procedures similar to that of G0-COOCH3 were used to produce 1.97 g (0.348 mmol) G2-COOCH3.Yield: 69.5%.
FTIR (KBr,cm-1): υ 2953,2873 (CH3,CH2),1738 (COOCH3),
1251 (Si-CH3),1041 (Si-O-Si); 1H NMR (CDCl3): δ -0.03 to 0.08
(m,174H,SiCH3),0.38-0.47 (m,84H,SiCH2CH2CH2Si),0.51-0.62
(m,24H,SiCH2CH2CH2N),1.24-1.34 (m,42H,SiCH2CH2CH2Si),
1.40-1.43 (m,24H,SiCH2CH2CH2N),2.37-2.46 (m,72H,
SiCH2CH2CHvNCH2),2.74-2.79 (t,48H,NCH2CH2CO),3.65 (s,72
H,OCH3/sub>); HRMS (FAB): m/z 5660.1801 [MH]+ (calcd. 5659.1722).
3. Results and discussion
In general,the syntheses of carbosilane dendrimers with
different peripheral functional groups were commonly achieved
by the divergent method. Based on this strategy,ester-capped
carbosilane dendrimers could also be synthesized according to the
procedures described in Scheme 2 (G0-COOCH3 was chosen as a
representative). As shown in Scheme 2,G0-NH2 can be first
synthesized according to the procedures that described in our
previous studies [11, 13],and then the Michael addition of MA to
the -NH2 group in G0-NH2 leads to the formation of G0-COOCH3
[14]. However,the divergent synthesis strategy can be hindered by
side reactions that yield incomplete or imperfect dendrimers
[13, 15]. During the hydrosilylation process of ATMS with MHMH
and G0 with MHM-ATMS,the amino groups should be protected by
a trimethylsilyl group to reduce the side reactions between the
amino group and the silane. An alcoholysis reaction should be
employed to deprotect the silyl group in order to obtain G0-NH2
[11]. Moreover,ATMS must be added in excess to ensure the
completion of the reaction with MHMH due to its relative low
activity. To overcome these drawbacks and the tedious protection-
deprotection procedures,a hybrid divergent-convergent method
was adopted. As illustrated in Scheme 1,G0,G1,and G2 were first
synthesized by the divergent method,then the MHM-DMAA was
convergently attached to the allyl group in G0-G2 to give the
corresponding G0-COOCH3-G2-COOCH3. Using this method,
DMAA was obtained by the Michael addition of MA with AA in
nearly 100% yield [14]. The intermediate ester-capped compound
MHM-DMAA was prepared by the hydrosilylation of DMAA with
MHMH in the presence of the Karstedt catalyst in excellent yield
(94.1%). At last,G0-COOCH3-G2-COOCH3 can be produced by a
hydrosilylation reaction of MHM-DMAA with G0,G1,and G2 in
good yields (78.0%,72.1%,and 69.5%,respectively). Therefore,the
hybrid divergent-convergent method provides simpler synthetic
procedures and higher yields compared to that of the divergent
method,and was suitable for the synthesis of ester-capped carbosilane dendrimers.
In order to ensure the hydrosilylation reaction proceeds readily
and completely,excess MHM-DMAA was used in the reaction. In addition,the content of C=C groups and the steric hindrance of the
dendrimer increase in later generations,and thus the amount of
MHM-DMAA,the Karstedt catalyst,and the reaction time need to
be increased to complete the reaction. Previous studies showed
that allyl derivatives allowed the synthesis of carbosilane
dendrimers up to the fifth generation with unified molecular
structures [16]. Montealegre also successfully synthesized the
third generation carboxylate-terminated carbosilane dendrimers
possessing 32 terminal groups with a molecular weight of 6586
[17]. The structures of G0-COOCH3-G2-COOCH3 and the intermediate
compounds were confirmed by FTIR,1H NMR,and HRMS
analyses. The FITR spectrum of MHM-DMAA showed the
characteristic adsorption bands of υ(Si-H) and υ(COOCH3) at
2110 and 1738 cm-1,and the adsorption bands at 1251 and
1041 cm-1 corresponding to δ(Si-CH3) and υ(Si-O-Si),respectively.
After the hydrosilylation reaction of MHM-DMAA with
G0-G2,the vibration peaks of υ(Si-H) at 2110 cm-1 and υ(C=C) at
1630 cm-1 disappeared in the FTIR spectrum of G0-COOCH3-G2-
COOCH3,and new adsorption peaks at 1041 and 1738 cm-1
attributed to υ(Si-O-Si) and υ(COOCH3) appeared,indicating that G0-COOCH3-G2-COOCH3 had been successful synthesized. The 1H
NMR spectrum of G0-COOCH3 exhibited resonances for Si-CH3
protons in the region of 0.00-0.10 ppm and for ester protons
(OCH3) at 3.65 ppm. The resonances appeared in the range of 0.38-
0.48,0.50-0.59,1.24-1.45,2.36-2.53,and 2.73-2.91 ppm can be
assigned to the protons of SiCH2CH2CH2Si,SiCH2CH2CH2N,
SiCH2CH2CH2Si,SiCH2CH2CH2NCH2,and NCH2CH2CO,respectively.
The 1H NMR spectrum of G1-COOCH3 and G2-COOCH3 are almost
identical to that of G0-COOCH3 for analogous protons,although
broader resonances are observed with the increased generations.
The reason for this can be ascribed to the slightly different
chemical environments for the protons in different generations
and the restricted mobility of the respective protons in the outer
shells [14]. In the 1H NMR spectrum of G0-COOCH3-G2-COOCH3,
the ratio of ester end groups (OCH3) to CH3Si groups was consistent
with the expected value based on the formula. Also,the complete
disappearance of C=C group resonances in the spectrum of G0-G2
further confirmed the formation of unified molecular dendrimers
of G0-COOCH3-G2-COOCH3. Furthermore,the molecular weights
of G0-COOCH3-G2-COOCH3 were determined by HRMS,which
were in good agreement with the theoretical calculation. This
unambiguously confirmed the formation of G0-COOCH3-G2-
COOCH3,further demonstrating that the desired ester-capped
carbosilane dendrimers had been successfully synthesized.
4. Conclusion
In conclusion,a series of novel ester-capped carbosilane
dendrimers (G0-COOCH3-G2-COOCH3) were successfully synthesized
via a hybrid divergent-convergent method in good yield. All
the targeted dendrimers were confirmed by FTIR,1H NMR,and HRMS analyses. The synthesized dendrimers possess reactive ester functional groups,which can be further functionalized using appropriate synthetic procedures to expand the application of
carbosilane dendrimers.
Acknowledgments
The authors are grateful for the financial support by the
National Natural Science Foundation of China (No. 21307053),
China Postdoctoral Science Foundation Funded Project (No.
2013M541911),Promotive Research Fund for Excellent Young
and Middle-Aged Scientists of Shandong Province (No.
BS2013CL044),and Natural Science Foundation of Ludong
University (No. LY2011004).
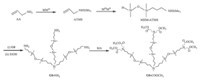
Scheme 2. The divergent synthesis routes of G0-COOCH3.
| [1] | Y.J. Pu, H. Yuan, M. Yang, B. He, Z.W. Gu, Synthesis of peptide dendrimers with polyhedral oligomeric silsesquioxane cores via click chemistry, Chin. Chem. Lett. 24 (2013) 917-920. |
| [2] | Y. Zhang, M.Y. Xu, T.K. Jiang, et al., Low generational polyamidoamine dendrimers to enhance the solubility of folic acid: a "dendritic effect" investigation, Chin. Chem. Lett. 25 (2014) 815-818. |
| [3] | P. Kesharwani, K. Jain, N.K. Jain, Dendrimer as nanocarrier for drug delivery, Prog. Polym. Sci. 39 (2014) 268-307. |
| [4] | J.P. Majoral, A.M. Caminade, Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi), Chem. Rev. 99 (1999) 845-880. |
| [5] | S.G. Zhao, C.J. Zhou, J.M. Zhang, J. Wang, S.Y. Feng, Investigation of allyl-capped carbosilane dendrimers used as crosslinker for silicone rubber, J. Appl. Polym. Sci. 100 (2006) 1772-1775. |
| [6] | D.Y. Son, A durable template for carbosilane dendrimer synthesis, Chem. Commun. 49 (2013) 10209-10210. |
| [7] | A.J. Perisé -Barrios, J.L. Jimé nez, A. Domínguez-Soto, et al., Carbosilane dendrimers as gene delivery agents for the treatment of HIV infection, J. Control. Release 184 (2014) 51-57. |
| [8] | M. Galá n, J.S. Rodríguez, J.L. Jimé nez, et al., Synthesis of new anionic carbosilane dendrimers via thiolene chemistry and their antiviral behavior, Org. Biomol. Chem. 12 (2014) 3222-3237. |
| [9] | L.L. Zhou, J. Roovers, Synthesis of novel carbosilane dendritic macromolecules, Macromolecules 26 (1993) 963-968. |
| [10] | B. Marciniec, Hydrosilylation: A Comprehensive Review on Recent Advances, 1st ed., Springer-Verlag Inc., New York, 2009, 226 pp.. |
| [11] | C.F. Li, D.X. Li, S.Y. Feng, Synthesis of platinum-terminated dendritic carbosilane, Polym. Int. 54 (2005) 1041-1046. |
| [12] | W.Y. Sun, D.X. Wang, S.Y. Feng, Synthesis of carbosilane dendrimers with N,Ndimethylaniline as end groups, Chin. Chem. Lett. 20 (2009) 300-301. |
| [13] | W.Y. Sun, H.F. Lu, D.X. Wang, S.Y. Feng, A series of carbosilane dendrimers with acetyl end-group: from synthesis to their unique optical characteristics, Chin. Chem. Lett. 20 (2009) 1085-1087. |
| [14] | Y.Z. Niu, H.F. Lu, D.X. Wang, Y.Z. Yue, S.Y. Feng, Synthesis of siloxane-based PAMAM dendrimers and luminescent properties of their lanthanide complexes, J. Organomet. Chem. 696 (2011) 544-550. |
| [15] | S.H. Medina, M.E.H. El-Sayed, Dendrimers as carriers for delivery of chemotherapeutic agents, Chem. Rev. 109 (2009) 3141-3157. |
| [16] | A.W. van der Made, P.W.N.M. van Leeuwen, Silane dendrimers, J. Chem. Soc. Chem. Commun. (1992) 1400-1401. |
| [17] | C. Montealegre, B. Rasines, R. Gó mez, et al., Characterization of carboxylateterminated carbosilane dendrimers and their evaluation as nanoadditives in capillary electrophoresis for vegetable protein profiling, J. Chromatogr. A 1234 (2012) 16-21. |




