Enantioseparation of chiral compounds is attracting burgeoning interests currently, which is partly attributed to the significant differences in biological, pharmacological and toxicological effects of enantiomers [1]. To date, high-performance liquid chromatography (HPLC) is the most commonly used method for chiral separation of drug and pesticide enantiomers and a variety of chiral selectors are available [2]. Among them, the functionalized cyclodextrin (CD) has attracted much attention because of its effective stereoisomer separation abilityviathe mechanism of forming inclusion complexes with the analytes. From the practical standpoints, the most popular CSPs are based on the β-cyclodextrin [3], which is an oligosaccharide of seven glucose units cyclized together to form a toroidal structure with a hydrophilic exterior face and a hydrophobic inner cavity. Since Armstrong [4] developed the first high efficiency β-CD bonded phase using 5mm silica gel as the packing material for HPLC, numerous compounds have been chirally separated using the technique. Particularly, more and more attention has been paid to the dimethylatedβ-CD bonded phase, which could minimize the hydrogen bonding interactions and rely more on the weaker dipole effects to separate the structural and positional isomers in a reversed-phase system.
Previously, research efforts were directed mainly to chemically anchoring cyclodextrin onto solid matriceviaether [5], amino [6], or triazine spacers [7], or the solvolytically more stable urea [8] or carbamate linkages [9]. These approaches are not satisfactory because they involve the use of some expensive raw materials and some toxic, explosive or metal-containing catalysts. Furthermore, the preparation processes mostly involve the protection and deprotection of hydroxyl groups in the cyclodextrins, which make the synthetic routes tedious and complex. These factors, in part, may have contributed to the high cost and poor availability of the CD–CSP. 2. Experimental
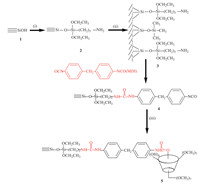
In this study, we report a novel approach to synthetize a 2,6-dimethyl-β-CD bonded stationary phase onto silica gel, which effectively overcomes the above shortcomings. The synthetic route to the new CSP is depicted in Scheme 1 using the 5–8mm totally porous silica microspheres 1(Fig. S1 in Supporting information) as the starting material. These silica microspheres with a relatively great BET surface area (278 m2/g) and a single BET pore-size distribution (8 nm) were prepared in the author’s laboratoryviathe polymerization-induced colloid aggregation (PICA) method [10]. The colloidal silica (30–50 nm) needed in this process was made fromsilica powder (10–30mm, 99.9%), which could ensure the high purity of HPLC column packing. First, the aminized silica gel 2 was prepared by stirring the silica gel 1(dried overnight at 120℃ under 0.1 MPa vacuum) with γ-aminopropyltriethoxysilane in toluene at 100℃ for 4 h. Then, the residual silicon-hydroxyl bonds were protected by trimethylchlorosilane at 50℃ for 4 h. Thereafter, in order to enhance the chiral recognition performance, we introduced the commercially available diphenylmethane diisocyanate (MDI), which could link the aminized silica with a stable urethane linkage. Finally, the dimethylated β-CD was easily immobilized on the other end of the spacer arm through a carbamate linkage. We anticipated that the π–π stacking of MDI could strengthen the chiral recognition ability. The last two reactions were carried out at 80℃ for 6 h under N2. To the best of our knowledge, we are among the first to use such a design and preparation.
|
Download:
|
|
Scheme 1.The preparation of 2,6-dimethyl-β-CD modified CSP. Reagents and
conditions: (Ⅰ) (EtO)3Si(CH2)3SiNH2/toluene/Δ; (Ⅱ) (CH3)3SiCl/toluene/Δ; (Ⅲ) 2,6-dimethyl-β-CD/toluene/N2/Δ. |
|
|
Download:
|
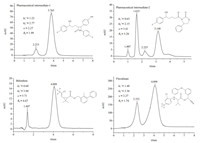
| Fig. 1.Representative chromatograms on the 2,6-dimethyl-β-CD modified CSP. | |
Due to the excellent solubility of 2,6-dimethyl-β-CD, the desired material 5 was conveniently purified and thoroughly characterized. As depicted in Fig. S2 in Supporting information, the immobilization is evident from the characteristic FT-IR vibrational bands, σ/cm-1 : 1602.0, 1515.5 (–Ph–); 1715.0 (– HNCOO–), 1554.4 (–CONH–). This result could be furtherσ corroborated by the obvious peaks in the solid-state 13C CP/MAS NMR spectrum (Fig. S3 in Supporting information),d/ppm: 0.6 (–Si(CH3)3); 9.4, 21.0, 41.7 (–CH2CH2CH2–); 60.5, 71.9, 80.9, 101.6 (–β-CD); 119.4, 127.3, 135.4, 147.2 (–Ph–), 162.7 (–NHCO–) and the solid-state 29Si CP/MAS NMR spectrum (Fig. S4 in Supporting information), δ/ppm: -109.9, -102.1 (–Si–O–Si–, Q4,Q3); -66.9,-58.4 (–Si–O–Si–C–, T3); 8.7 (–Si–C–, T1). In addition, the carbon content in the elemental analysis (C, 11.83%; H, 2.24%; N, 2.177%) of the CSP also shows that the dimethylated β-CD moieties have been successfully immobilized onto the surface of the silica gel. According to the microanalytical data, the surface concentration of the cyclodextrin derivatives on the silica gel is calculated to be 0.36mmol/m2.
After being packed into a stainless HPLC column (4.6×150 mm) via the slurry method, the resultant CSP exhibite excellent enantioseparation ability toward a wide range of structurally diverse chiral pesticide and drug compounds. The representative chromatograms are summarized in Fig. 1. It is evident that good selectivity factors (α) as well as resolutions (Rs) and short retentions (κ) are achieved in less than 10 min. This indicates that the current synthetic procedures can be used to afford improved CSPs. Further investigations on the detailed chromatographic behaviors as well as the separation mechanism involved will be reported elsewhere. 4. Conclusion
This paper describes the preparation process and chromatographic characteristics of a novel 2,6-dimethyl-β-CD bonded and silica based HPLC chiral stationary phase (CSP). The diphenylmethane diisocyanate (MDI) was applied for the first time in the immobilization process under mild conditions. The representative chromatographic properties demonstrate that this kind of CSP has good chiral separation ability for a variety of chiral compounds under reversed-phase conditions.
| [1] | G. Varga, G. Fodor, I. Ilisz, et al., Comparison of separation performances of novel b-cyclodextrin-based chiral stationary phases in high-performance liquid chromatographic enantioseparation, J. Pharm. Biomed. Anal. 70 (2012) 71-76. |
| [2] | R.N. Rao, M.V.N. Talluri, P.K. Maurya, Separation of stereoisomers of sertraline and its related enantiomeric impurities on a dimethylated b-cyclodextrin stationary phase by HPLC, J. Pharm. Biomed. Anal. 50 (2009) 281-286. |
| [3] | D.D. Schumacher, C.R. Mitchell, T.L. Xiao, et al., Cyclodextrin-based liquid chromatographic enantiomeric separation of chiral dihydrofurocoumarins, an emerging class of medicinal compounds, J. Chromatogr. A 1011 (2003) 37-47. |
| [4] | Armstrong D.W. Bonded phase material for chromatographic separations, U.S. Patent 4,539,399, 1985. |
| [5] | X.P. Chen, Z.M. Zhou, H. Yuan, Z.H. Meng, Preparation and chiral recognition of a novel chiral stationary phase for HPLC, based on mono (6A-N-1-(2-hydroxyl)- phenylethylimino-6A-deoxy)-β-cyclodextrin and covalently bonded silica gel, Chin. Chem. Lett. 19 (2008) 797-800. |
| [6] | (a) L.F. Zhang, Y.C. Wong, L. Chen, C.B. Ching, S.C. Ng, A facile immobilization approach for perfunctionalised cyclodextrin onto silica via the Staudinger reaction, Tetrahedron Lett. 40 (1999) 1815-1818; (b) X.H. Lai, S.C. Ng, Mono(6A-N-allylamino-6A-deoxy)perphenylcarbamoylated b-cyclodextrin: synthesis and application as a chiral stationary phase for HPLC, Tetrahedron Lett. 44 (2003) 2657-2660; (c) X.H. Lai, S.C. Ng, Convenient synthesis of mono(6A-N-allylamino-6A-deoxy) permethylated b-cyclodextrin: a promising chiral selector for an HPLC chiral stationary phase, Tetrahedron Lett. 44 (2004) 4469-4472; (d) X.H. Lai, W.H. Tang, S.C. Ng, Novel b-cyclodextrin chiral stationary phases with different length spacers for normal-phase high performance liquid chromatography enantioseparation, J. Chromatogr. A 1218 (2011) 3496-3501. |
| [7] | (a) Y.Y. Zhao, Z.M. Guo, Y.P. Zhang, Retention properties of novel b-CD bonded stationary phases in reversed-phase HPLC mode, Talanta 78 (2009) 916-921; (b) Y. Wang, D.J. Young, T.T.Y. Tan, S.C. Ng, "Click" preparation of hindered cyclodextrin chiral stationary phases and their efficient resolution in high performance liquid chromatography, J. Chromatogr. A 1217 (2010) 7878-7883. |
| [8] | (a) S.C. Ng, L. Chen, L.F. Zhang, et al., Facile preparative HPLC enantioseparation of racemic drugs using chiral stationary phases based on mono-6A-azido-6A-deoxyperphenylcarbamoylated b-cyclodextrin immobilized on silica gel, Tetrahedron Lett. 43 (2002) 677-681; (b) G. Varga, G. Tárká nyi, K. Németh, et al., Chiral separation by a monofunctionalized cyclodextrin derivative: from selector to permethyl-β-cyclodextrin bonded stationary phase, J. Pharm. Biomed. Anal. 51 (2010) 84-89. |
| [9] | K. Nakamura, H. Fujima, H. Kitagawa, et al., Preparation and chromatographic characteristics of a chiral-recognizing perphenylated cyclodextrin column, J. Pharm. Biomed. Anal. 694 (1995) 111-118. |
| [10] | (a) L. Sun, M.J. Annen, F. Lorenzano-Porras, et al., Synthesis of porous zirconia spheres for HPLC by polymerization-induced colloid aggregation (PICA), J. Colloid Interface Sci. 163 (1994) 464-473; (b) Z.T. Jiang, Y.M. Zuo, Synthesis of porous titania microspheres for HPLC packings by polymerization-induced colloid aggregation (PICA), Anal. Chem. 73 (2001) 686-688. |




