It is well known that CO2 is the most important component of greenhouse gases resulting in global warming [1]. During the past few years,the increasing burning of fossil fuels and petroleum products has led to the increasing concentration of CO2 in the atmosphere. The adjustment of global atmospheric circulation and the extension of climate zones to polar regions has caused climate anomalies and natural disasters [2]. Moreover,global warming will also cause and aggravate epidemics. Thus,capturing CO2 from fossil fuel exhaust fumes effectively and separating CO2 from the atmosphere is imperative. Conventionally,the most widely adopted approach to absorb CO2 uses aqueous organic amine solutions as chemical sorbents at fossil fuel-burning power plants, but severe economic burden and corrosion to the production plant are very cumbersome [3]. As an alternative method,physical adsorption by porous solids such as silicates,activated carbons, microporous and mesoporous inorganic molecular sieves,metalorganic frameworks (MOFs) [4, 5, 6, 7],and porous organic networks has attracted much attention for improving the drawbacks of amine-based chemical absorption processes. However,due to the disadvantages of most of these porous materials,for instance,complex synthetic process,weak stability,and lack of interaction between the pore surface and CO2 molecule,new kinds of materials also need to be developed.
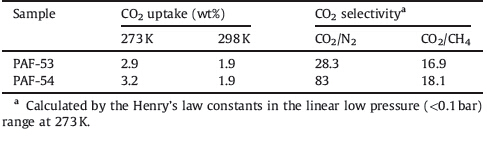
Porous organic networks including hypercrosslinked polymers (HCPs) [8],polymers with intrinsic micro-porosity (PIMs) [9, 10, 11], conjugated microporous polymers (CMPs) [12],covalent triazinebased frameworks (CTFs) [13],porous polymer networks (PPN) [14],and porous aromatic frameworks (PAFs) [15, 16, 17],which are only composed of organic components,usually have low skeletal density,high surface areas,and high stability,leading to higher unit mass sorption in gas capture operations. Interactions between CO2 and the PAF network exist at two points that may be modified during the construction of the PAF: (a) π-electron system of aromatic frameworks with triazine and the C atom of CO2; (b) potential between exposed nitrogen atoms from triazine rings and the C atom of CO2. Based on these strategies to increase interactions between CO2 and porous organic networks,we chose electron-rich triazine as the building unit due to its nitrogen content. So we chose cyanuric chloride as a triazine supplier,and an amino compound (p-phenylenediamine and melamine) would also offer nitrogen atoms in the framework. We synthesized novel porous aromatic frameworks (PAF-53 and PAF-54) using amino compound (p-phenylenediamine and melamine) and cyanuric chloride via a facile reaction (Fig. 1). They possess high thermal stability. Interestingly,they show high selectivity of CO2/CH4 and CO2/N2.
|
Download:
|
| Fig. 1. Scheme of PAFs synthesis reaction: (a) p-phenylenediamine as reagent; (b) melamine as reagent. | |
All starting materials were purchased from commercial suppliers and used without further purification unless otherwise noted. Cyanuric chloride was purchased from Alfa Aesar; pphenylenediamine and melamine were purchased from Aladdin Industrial Corporation. 2.2. Synthesis of PAF-53
The p-phenylenediamine (108mg,1mmol) and dimethylacetylamide (DMAc) (10 mL) were mixed well and N,N-diisopropylethylamine (DIPEA) (1.5 mL,8.6mmol) was added to the mixture under nitrogen. After cooling to 0 ℃,15 mL of cyanuric chloride solution inDMAc (184.5mg,1mmol) were added dropwise into the mixture within 30 min under stirring. Then the reaction solution was heated at 50 ℃ for 12 h and then at 95 ℃ for 24 h. After removal of most of the solvent under reduced pressure,the product was extracted with THF in a Soxhlet apparatus over at least 48 h. 2.3. Synthesis of PAF-54
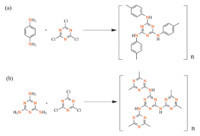
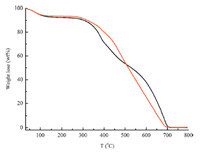
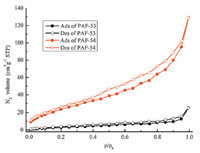
The melamine (126 mg,1 mmol) and dimethylacetylamide (DMAc) (10 mL) were mixed well and N,N-diisopropylethylamine (DIPEA) (1.5 mL,8.6 mmol) was added to the mixture under nitrogen. After cooling to 0 ℃,15 mL of cyanuric chloride solution in DMAc (184.5 mg,1 mmol) were added dropwise into the mixture within 30 min under stirring. Then the reaction solution was heated at 50 ℃ for 12 h and then at 95 ℃ for 24 h. After removal of most of the solvent under reduced pressure,the product was extracted with THF in a Soxhlet apparatus over at least 48 h. 2.4. Characterization The FTIR spectra were measured by using a Nicolet Impact 410 Fourier transform infrared spectrometer. Samples were pestled and packed firmly to get transparent films. The powder X-ray diffraction (PXRD) was performed by a Rigaku D/max 2500 diffractometer using CuKα radiation at 40 kV,200 mA,with scanning rate of 0.3° min-1 (2θ). The thermogravimetric analysis (TGA) was performed by using a NetzchSta449c thermal analyzer system at the heating rate of 10 ℃ min-1 under air atmosphere. Scanning electron microscopy (SEM) was recorded by using a JEOLJEM 6700. Gas sorption isotherms were recorded on an Autosorb-Q1 surface area and pore size analyzer. The samples were degassed at 393 K for 12 h (3 × 10-3 Torr) prior to adsorption measurements. UHP grade (99.999%) N2,Ar,CO2,and CH4 were used for all measurements. The temperatures were maintained at 77 K in liquid nitrogen bath,273 K in ice-water bath,and 298 K in water bath,respectively. The specific surface areas of the samples were calculated with the BET method. 3. Results and discussion FTIR spectra (Fig. 2) of initial monomers and final products provide us with information of the reaction process. The disappearance of N-H bands of primary amines at 1634 cm-1 (PAF-53) and 1650 cm-1 (PAF-54) clearly indicated the substitution of an amine group in the amino compound reagent. Thermogravimetric analysis (TGA) under air condition of the two samples revealed a high thermal stability up to 300 ℃ (Fig. 3). Powder X-ray diffraction (PXRD) of these PAFs revealed no distinct diffraction peaks (Fig. 4),indicating that these polymers are composed of an amorphous network. Scanning electron microscopy (SEM) analyses in Fig. 5 display that the polymers consist of aggregated irregular lumps. The N2 sorption isotherms were measured at 77 K to prove the porous properties of PAF materials (Fig. 6). The surface area of PAF-53 is nearly zero and the surface area of PAF-54 is 93.2 m2 g-1 based on the BET model.

|
Download:
|
| Fig. 2. (a) FTIR spectra of PAF-53 (red) and p-phenylenediamine (black); (b) FTIR spectra of PAF-54 (red) and melamine (black). | |
|
Download:
|
| Fig. 3. TGA plots for two polymer networks PAF-53 (black) and PAF-54 (red) at air condition with the rate of 10 ℃ min-1. | |
|
Download:
|
| Fig. 4. The PXRD patterns of PAF-53 (a) and PAF-54 (b). | |

|
Download:
|
| Fig. 5. SEM images of PAF-53 (a) and PAF-54 (b). | |
|
Download:
|
| Fig. 6. N2 sorption isotherms of PAF-53 (black),PAF-54 (red) (filled: adsorption; hollow: desorption). | |
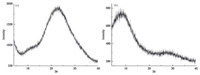
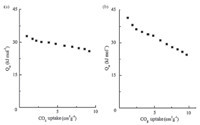
We have plotted the CO2 adsorption isotherms of the two PAFs at 273 K and 298 K respectively (Fig. 7). A rapid rise at the initial stage indicates that there might be favorable interactions between CO2 molecules and PAF networks,and much higher storage capacity can be expected at increased pressure for the unsaturation in the measurement range. The total gravimetric uptake of PAF-53 is 2.9 wt% at 1 bar and 273 K. The absorbance also can reach 1.9 wt% at room temperature (298 K). For PAF-54 under 273 K and 298 K,the absorbances were 3.2 and 1.9 wt% at 1 bar,respectively. These results indicate that PAF-53 and PAF-54 have comparable CO2 sorption capacity. As in Fig. 8,PAF-53 showed a heat of adsorption (Qst) of 32.8 kJ mol-1 at zero-loading,while that for PAF-54 is 41.5 kJ mol-1. Compared with some acid-functionalized porous polymers,for example,PPN-6-SO3H (30.4 mol-1) [18], CMP-1-COOH (32.6 kJ mol-1) [12],PAF-18-OH,and PAF-18-OLi (28.0 and 29.5 mol-1) [19],the Qst of PAF-53 and PAF-54 is substantially higher. The high Qst value of PAFs should be due to the highly polar framework with high content of N atoms that facilitate the strong dipole-quadrupole interactions between the pore surface and CO2.

|
Download:
|
| Fig. 7. CO2 sorption isotherms of PAF-53 (a) and PAF-54 (b) at 273 K and 298 K. | |
|
Download:
|
| Fig. 8. Calculated isosteric heats of adsorption for CO2 uptake of PAF-53 (a) and PAF-54 (b). | |
Owing to the nitrogen-rich frameworks in PAFs,CO2 should have higher adsorption compared with other gases such as N2 and CH4. When comparing CO2 and N2 under 298 K,adsorption for CO2 is much higher than for N2. As shown in Table 1,the selectivity of PAF-54 reaches 83,which was calculated from the initial slopes of the CO2 and N2 adsorption isotherms,which is better than many other porous materials with higher surface area and CO2 uptakes [20]. Likewise,the selective adsorption of CO2 to CH4 reaches 18.1 at 273 K,which can be compared with other porous materials which also uses nitrogen-rich organic building blocks,such as zeolite-like zinc-tetrazole Framework reported by Hongcai Zhou with a separation factor of 21.1 [7]. These remarkable features can be used for cleaning renewable energy which still needs further study. The prominent selective adsorption of CO2 might be ascribed to the high nitrogen content in the networks,which offers electron-rich condition and enhances the interaction with CO2 molecules,leading to their selective adsorption over other gases.
| Table 1 CO2 uptake and selectivity of PAF-53 and PAF-54. |
In summary,we have successfully synthesized nitrogen-rich PAFs with p-phenylenediamine/melamine and cyanuric chloride. The products are thermally stable and possess a certain amount of CO2 sorption capacity. Furthermore,selectivity of CO2 is as high as 83 over N2 and 18.1 over CH4 at room temperature for PAF-54. Acknowledgments
We are grateful for the financial support of the National Natural Science Foundation of China (No. 0831002),Major International (Regional) Joint Research Project (No. 21120102034).
| [1] | R.S. Haszeldine, Carbon capture and storage: how green can black be? Science 325 (2009) 1647-1652. |
| [2] | D. Leaf, H.J.H. Verolme, W.F. Hunt Jr., Overview of regulatory/policy/economic issues related to carbon dioxide, Environ. Int. 29 (2003) 303-310. |
| [3] | G.T. Rochelle, Amine scrubbing for CO2 capture, Science 325 (2009) 1652-1654. |
| [4] | B. Li, Z. Zhang, Y. Li, et al., Enhanced binding affinity, remarkable selectivity, and high capacity of CO2 by dual functionalization of a rht-type metal-organic framework, Angew. Chem. Int. Ed. 51 (2012) 1412-1415. |
| [5] | K. Sumida, D.L. Rogow, J.A. Mason, et al., Carbon dioxide capture in metal organic frameworks, Chem. Rev. 112 (2012) 724-781. |
| [6] | Z.Q. Liang, J.J. Du, L.B. Sun, et al., Design and synthesis of two porous metal-organic frameworks with nbo and agw topologies showing high CO2 adsorption capacity, Inorg. Chem. 52 (2013) 10720-10722. |
| [7] | P. Cui, Y.G. Ma, H.H. Li, et al., Multipoint interactions enhanced CO2 uptake: a zeolite-like zinc-tetrazole framework with 24-nuclear zinc cages, J. Am. Chem. Soc. 134 (2012) 18892-18895. |
| [8] | J.Y. Lee, C.D. Wood, D. Bradshaw, M.J. Rosseinsky, A.I. Cooper, Hydrogen adsorption in microporous hypercrosslinked polymers, Chem. Commun. (2006) 2670- 2672. |
| [9] | P.M. Budd, E.S. Elabas, B.S. Ghanem, et al., Solution-processed, organophilic membrane derived from a polymer of intrinsic microporosity, Adv. Mater. 16 (2004) 456-459. |
| [10] | N.B. McKeown, B. Ghanem, K.J. Msayib, et al., Towards polymer-based hydrogen storage materials: engineering ultramicroporous cavities within polymers of intrinsic microporosity, Angew. Chem. Int. Ed. 45 (2006) 1804-1807. |
| [11] | N.Y. Du, G.P. Robertson, J.S. Song, et al., Polymers of intrinsic microporosity containing trifluoromethyl and phenylsulfone groups as materials for membrane gas separation, Macromolecules 41 (2008) 9656-9662. |
| [12] | R. Dawson, D.J. Adams, A.I. Cooper, Chemical tuning of CO2 sorption in robust nanoporous organic polymers, Chem. Sci. 2 (2011) 1173-1177. |
| [13] | P. Kuhn, M. Antonietti, A. Thomas, Porous, covalent triazine-based frameworks prepared by ionothermal synthesis, Angew. Chem. Int. Ed. 47 (2008) 3450-3453. |
| [14] | D. Yuan, W. Lu, D. Zhao, H.C. Zhou, Highly stable porous polymer networks with exceptionally high gas-uptake capacities, Adv. Mater. 23 (2011) 3723-3725. |
| [15] | H. Zhao, Z. Jin, H.M. Su, et al., Target synthesis of a novel porous aromatic framework and its highly selective separation of CO2/CH4, Chem. Commun. 47 (2011) 2780-2782. |
| [16] | Y. Yuan, F.X. Sun, H. Ren, et al., Targeted synthesis of a porous aromatic framework with a high adsorption capacity for organic molecules, J. Mater. Chem. 21 (2011) 13498-13502. |
| [17] | W. Wang, H. Ren, F.X. Sun, et al., Synthesis of porous aromatic framework with tuning porosity via ionothermal reaction, Dalton Trans. 41 (2012) 3933-3936. |
| [18] | W.G. Lu, D.Q. Yuan, J.L. Sculley, et al., Sulfonate-grafted porous polymer networks for preferential CO2 adsorption at low pressure, J. Am. Chem. Soc. 133 (2011) 18126-18129. |
| [19] | H.P. Ma, H. Ren, X.Q. Zou, et al., Novel lithium-loaded porous aromatic framework for efficient CO2 and H2 uptake, J. Mater. Chem. A 1 (2013) 752-758. |
| [20] | R. Dawson, L.A. Stevens, T.C. Drage, et al., Impact of water coadsorption for carbon dioxide capture in microporous polymer sorbents, J. Am. Chem. Soc. 134 (2012) 10741-10744. |