In recent years,organic semiconductors have drawn significantly more attention,compared to the traditional inorganic materials such as amorphous or crystalline silicon. They displayed several advantages such as their adaptability to low-temperature processing on flexible substrates,low cost,amenability to highspeed fabrication,and tunable electronic properties [1, 2, 3]. Organic semiconductors become a promising technology for large-area electronic applications including electronic paper displays,radio frequency identification tags,smart cards,and large-area sensors [4, 5]. Organic semiconducting materials are commonly classified as p-type (hole-conducting) or n-type (electron-conducting),depending on which type of charge carrier is more efficiently transported through the material. So far,a large number of high performance p-type organic semiconductors have been studied,and stable organic p-type semiconductors have fulfilled many of the requirements in diverse applications. However,high performance n-type organic semiconductors are relatively rare,mainly owing to synthetic difficulties and poor air stability [6].
In view of the requirements for the fabrication of organic complementary circuits,n-type organic semiconductor has evoked increasing interest recently [7]. To afford air stable n-type organic semiconductors,the common strategy is to introduce strong electron-withdrawing groups to a conjugated system,such as cyano,imide or fluorine groups [8, 9]. Among all kinds of n-type organic semiconductors,naphthalene diimide (NDI) was considered one of the most promising candidates due to its good airstability and film-forming ability [10]. Fluorine atoms can lower both the HOMO and LUMO energy levels to facilitate the electron injection and stabilize the materials to display a greater resistance against the degradative oxidation processes [11]. Moreover,the C- H...F interactions play an important role in the solid state supramolecular organization,originating a typical π-stack arrangement that enhances the charge carrier mobility [12]. Herein,we report three fluorinated 1,8-napthalimides. Their structures were characterized and their optoelectronic properties were investigated. 2. Experimental
Compounds 3,4,5 and 6a have been reported in the literature [13, 14]. Unless otherwise mentioned,all commercially available starting materials were used as received without further purification. DMSO was dried with CaH2 and then distilled under reduced pressure. 1H NMR and 13C NMR spectra were collected on an American Varian Mercury Plus at 400 spectrometer 400 MHz or 600 MHz. Chemical shifts (δ) are reported in ppm,using TMS as an internal standard. MS spectra were collected on a Thermo DSQ II. UV-vis spectra were obtained on a U-3310 UV Spectrophotometer. Fluorescence spectra were obtained on a FluoroMax-P. The crystal structure was recorded on a Bruker SMART CCD area-detector Xray diffraction spectrometer. 2.1. General synthetic procedures for 6b and 6c
A mixture of 4,5-dibromo-1,8-naphthoic anhydride 5 (2.8 mmol),amine (2.8 mmol) in ethanol (50 mL) was stirred overnight under nitrogen at 78 ℃. The reaction mixture was then cooled and the solvent was removed under vacuum. The residue was purified by column chromatography using dichloromethane/ petroleum ether (1:3,v/v) as an eluent to afford pure product as a yellow solid.
Compound 6b. Yield: 0.49 g,40%. 1H NMR (400 MHz,CDCl3): δ 8.40 (d,2H,J= 5.6 Hz),8.21 (d,2H,J= 8 Hz),4.14 (t,2H,J= 7.6 Hz),1.73-1.68 (m,2H),1.41-1.25 (m,6H),0.89 (t,3H,J= 4 Hz). 13C NMR (100 MHz,CDCl3): δ 163.00,136.04,131.36,131.02,127.97,127.52,123.01,40.71,31.46,27.85,26.71,22.52,14.02. EI-MS: m/z 439.00; calcd. for C18H17Br2NO2: 439.14.
Compound 6c. Yield: 0.42 g,35%. 1H NMR (600 MHz,CDCl3): δ 8.38 (d,2H,J= 7.8 Hz),8.21 (d,2H,J= 8.4 Hz),5.01-4.96 (m,1H),2.24-2.17 (m,2H),1.93-1.87 (m,2H),0.89 (t,6H,J= 7.8 Hz). 13C NMR (100 MHz,CDCl3): δ 158.50,136.20,131.37,131.00,128.02,127.75,127.55,57.55,24.10,10.90. EI-MS: m/z 424.82; calcd. for C17H15Br2NO2: 424.94. 2.2. General synthetic procedures for 1a-c
A mixture of 4,5-dibromo-1,8-naphthalimide (0.5 mmol),anhydrous powered CsF (3.3 mmol) in dry DMSO (50 mL) was stirred for 1 h under nitrogen at 95 ℃. The reaction mixture was then cooled and poured into ice and the yellow solid was collected by filtration. The residue was purified by column chromatography using dichloromethane/petroleum ether (1:3,v/v) as an eluent to afford the pure product.
Compound 1a. Yellow solid; yield: 36 mg,25%. 1H NMR (400 MHz,CDCl3): δ 8.63 (d,2H,J= 8.4 Hz),7.45-7.28 (m,2H),4.16 (t,2H,J= 7.6 Hz),1.72-1.68 (m,2H),1.25-1.46 (m,2H),0.97 (t,3H,J= 7.2 Hz). 13C NMR (100 MHz,CDCl3): δ 162.86,162.59,162.48,159.76,159.65,133.68,131.41,118.79,112.88,40.23,30.00,20.26,13.75. EI-MS: m/z 289.03; calcd. for C16H13F2NO2: 289.09.
Compound 1b. Yellow solid; yield: 33 mg,21%. 1H NMR (400 MHz,CDCl3): δ 8.63 (d,2H,J= 7.6 Hz),7.49-7.42 (m,2H),4.14 (t,2H,J= 7.6 Hz),1.75-1.67 (m,2H),1.41-1.33 (m,6H),0.89 (t,3H,J= 6.6 Hz). 13C NMR (100 MHz,CDCl3): δ 162.96,162.71,162.60,159.89,159.78,133.75,131.91,131.56,127.11,126.78,118.93,112.93,112.19,40.52,31.46,27.93,26.70,22.50,13.98. EIMS: m/z 317.18; calcd. for C18H17F2NO2: 317.12.
Compound 1c. White solid; yield: 22 mg,15%. 1H NMR (400 MHz,CDCl3): δ 8.61 (d,2H,J= 9.3 Hz),7.46-7.41 (m,2H),5.15-4.87 (m,1H),2.25-2.18 (m,2H),1.98-1.92 (m,2H),0.88 (t,6H,J= 7.4 Hz). 13C NMR (100 MHz,CDCl3): δ 162.56,162.43,133.85,131.74,119.21,119.03,113.02,112.91,112.81,57.48,24.52,10.82. EI-MS: m/z 303.09; calcd. for C17H15F2NO2: 303.10. 3. Results and discussion
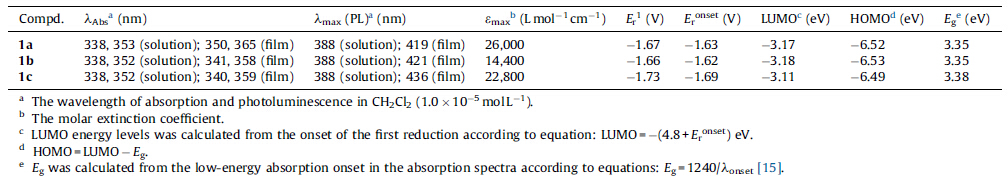
The synthesis of 1a-c was outlined in Scheme 1. Acenaphthene was bromated using NBS to give 3. Subsequently,a two-step oxidation reaction was performed to afford 5. Then naphthoic anhydride was treated with different amine in ethanol to afford the corresponding bromo-1,8-naphthalimide. The targeted compounds were prepared from bromo-1,8-naphthalimide in dry DMSO using CsF. These compounds showed very good solubility in common solvents,such as dichloromethane,chloroform and acetone. This good solubility allowed them to be processed in solution and allowed the formation of thin films. The optical properties were investigated by UV-vis absorption and fluorescence spectrometry. Fig. 1a showed the UV-vis absorption and fluorescence spectra of 1a in CH2Cl2. Two characteristic absorption peaks at 338 nm and 353 nm were observed,compared with the absorption bands at 334 nm and 344 nm of non-substituted 1,8- naphthalimide [16],which suggested that the introduction of the two fluorine atoms led to a slight red shift. Compound 1a had a wavelength of 388 nm for the maximum fluorescence emission in solution in Fig. 1a and Table 1,which represented a 50 nm Stokes shift (excited at 338 nm).

|
Download:
|
| Scheme 1.Synthesis of compounds 1a-c. | |
|
Download:
|
| Fig. 1. (a) Normalized UV-vis absorption and photoluminescence spectra of 1a in CH2Cl2 (1.0 × 10-5 mol L-1) and in thin film; (b) 1b in CH2Cl2 (1.0 × 10-5 mol L-1) and in thin film; (c) 1c in CH2Cl2 (1.0 × 10-5 mol L-1) and in thin film; (d) Cyclic voltammogram (CV) of 1a-c in dry DCM with 0.1 mol L-1 Bu4NPF6 as supporting electrolyte. | |
| Table 1 Optical and electrochemical data of 1a-c. |
The UV-vis absorption and fluorescence spectra of 1a were also measured in the thin-film state as shown in Fig. 1a. The UV-vis absorption spectra and fluorescence spectra collected in the thin films were broader than those spectra collected in solutions and showed a large bathochromic shift,indicating that the chromophore had a strong tendency to aggregate in the solid state.
As can be seen in Fig. 1b and c and Table 1,similar phenomena were also observed on the absorption and emission spectra of 1b and 1c in the solution and in the film state,indicated that the different alkyl chain had little effects on their optical properties. The values of optical energy gaps (Eg) (~3.35-3.38 eV) were calculated from the low-energy absorption onset in the absorption spectra,as shown in Table 1.
Subsequently,the electrochemical properties of these naphthalimides were investigated by cyclic voltammetry (CV). The measurements were carried out in dry dichloromethane at room temperature under nitrogen atmosphere. As shown in Fig. 1d,one pair of irreversible reduction waves were observed. For example,compound 1a showed a reduction wave at -1.67 V and no obvious oxidation waves were observed. The LUMO energy level of -3.17,-3.18,-3.11 eV were estimated for 1a-c based on the onset potential of the first reduction wave. The HOMO energy levels of 1a-c were also calculated based on the equation described in Table 1. From these calculations,we found that these compounds had similar HOMO and LUMO energy levels. The HOMO and LUMO energy levels of 1a-c were much lower,in comparison with those of non-substituted 1,8-naphthalimide. These data demonstrated that fluorine atoms lowered not only the LUMO energy level but also the HOMO energy level,which was significant to afford air stable n-type organic semiconductors.
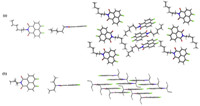
Single crystals of 1b and 1c suitable for crystallographic analysis were grown by slow diffusion of n-hexane to their dichloromethane solutions. As depicted in Fig. 2,C-H...O hydrogen bonds made 1b pack into one-dimensional planar structures. Compound 1c displayed lamellar packing promoted by the C-H...O hydrogen bonds and C-H...F hydrogen bonds. The slight structural change induced by alkyl chain resulted in an obvious change of packing form. It is well-known that molecular stacking is very important for the properties of a material [17]. Hence,these results afforded an alternative approach to explore organic optoelectronic materials by decorating of their structures.
|
Download:
|
| Fig. 2. The single crystal structures and packing views of 1b<> (a),1c (b). (Top,side,and packing view from left to right. Hydrogen atoms have been omitted in packing views.). | |
In summary,three fluorinated 1,8-napthalimides were successfully synthesized. The structures of compounds 1b and 1c were confirmed by single crystal X-ray diffraction analysis. It should be noted that compounds 1b and 1c had different packing motifs in the solid state. This work affords an alternative approach to the exploration of organic optoelectronic materials with good performance in devices,using only minor structural decorations. Their optoelectronic properties indicated that the introduction of fluorine atoms could lower the LUMO and HOMO energy level. Their good solubility and optoelectronic properties make them potential solution-processable candidates for organic devices.
Acknowledgments
We acknowledge financial support from National Natural Science Foundation of China (Nos. 21072070,21272088) and the Program for Academic Leader in Wuhan Municipality (No. 201271130441). The work was also supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars,Ministry of Education,the Natural Science Foundation of Hubei Province (No. 2013CFB207) and Excellent doctorial dissertation cultivation grant from Central China Normal University (No. 2013YBYB60).
| [1] | H. Usta, A. Facchetti, T.J. Marks, N-channel semiconductor materials design for organic complementary circuits, Acc. Chem. Res. 44 (2011) 501-510. |
| [2] | K.Y. Hua, C.M. Deng, C. He, et al., Organic semiconductors-coated polyacrylonitrile (PAN) electrospun nanofibrous mats for highly sensitive chemosensors via evanescent-wave guiding effect, Chin. Chem. Lett. 24 (2013) 643-646. |
| [3] | C. Zhan, Y.Y. Jiang, M.Y. Yang, L.H. Lu, S.Q. Xiao, Synthesis and optoelectronic properties of a novel molecular semiconductor of dithieno[5,6-b:11,12-b0] coronene-2,3,8,9-tetracarboxylic tetraester, Chin. Chem. Lett. 25 (2014) 65-68. |
| [4] | C. Di, F. Zhang, D. Zhu, Multi-functional integration of organic field-effect transistors (OFETs): advances and perspectives, Adv. Mater. 25 (2013) 313-330. |
| [5] | A.N. Sokolov, B.C.K. Tee, C.J. Bettinger, J.B.H. Tok, Z.N. Bao, Chemical and engineering approaches to enable organic field-effect transistors for electronic skin applications, Acc. Chem. Res. 45 (2012) 361-371. |
| [6] | Z. Bao, Materials and fabrication needs for low-cost organic transistor circuits, Adv. Mater. 12 (2000) 227-230. |
| [7] | Y.L. Lee, H.L. Hsu, S.Y. Chen, T.R. Yew, Solution-processed naphthalene diimide derivatives as n-type semiconductor materials, J. Phys. Chem. C 112 (2008) 1694-1699. |
| [8] | C.R. Newman, C.D. Frisbie, D.A. da Silva Filho, et al., Introduction to organic thin film transistors and design of n-channel organic semiconductors, Chem. Mater. 16 (2004) 4436-4451. |
| [9] | Y. Li, L. Tan, Z. Wang, et al., Air-stable n-type semiconductor: core-perfluoroalky-lated perylenebisimides, Org. Lett. 10 (2008) 529-532. |
| [10] | C. Wang, H. Dong, W. Hu, Y. Liu, D. Zhu, Semiconducting p-conjugated systems in field-effect transistors: a material odyssey of organic electronics, Chem. Rev. 112 (2012) 2208-2267. |
| [11] | J.L. Brédas, A.J. Heeger, Influence of donor and acceptor substituents on the electronic characteristics of poly(paraphenylene vinylene) and poly(paraphenylene), Chem. Phys. Lett. 217 (1994) 507-512. |
| [12] | F. Babudri, G.M. Farinola, F. Naso, R. Raqni, Fluorinated organic materials for electronic and optoelectronic applications: the role of the fluorine atom, Chem. Commun. (2007) 1003-1022. |
| [13] | H. Ge, X. Li, D. Wu, et al., Donor-acceptor naphthylimide: synthesis and properties, Mol. Cryst. Liq. Cryst. 582 (2013) 109-114. |
| [14] | M. Tesmer, H. Vahrenkamp, Sterically fixed dithiolate ligands and their zinc complexes: derivatives of 1,8-dimercaptonaphthalene, Eur. J. Inorg. Chem. 2001 (2001) 1183-1188. |
| [15] | C. Chi, G. Wegner, Chain-length dependence of the electrochemical properties of conjugated oligofluorenes, Macromol. Rapid Commun. 26 (2005) 1532-1537. |
| [16] | S. Erten-Ela, S. Ozcelik, E. Eren, Synthesis and photophysical characterizations of thermal-stable naphthalene benzimidazoles, J. Fluoresc. 21 (2011) 1565-1573. |
| [17] | L. Jing, H. Dong, W. Hu, Organic single crystal field-effect transistors: advances and perspectives, J. Mater. Chem. 20 (2010) 4994-5007. |