As a biodegradable compound,diethyl carbonate (DEC) has attracted much attention in recent years and has been widely used in organic synthesis,electrical chemistry,and fuel additives [1, 2, 3]. Currently,the existing methods for preparing DEC mainly include phosgenation of ethanol,oxidative carbonylation of ethanol,transesterification of carbonate and ethanolysis of CO2 [4, 5, 6]. However,these methods suffer from drawbacks such as high toxicity,inconvenient operations,complicated processes and poor yields,which were undesirable for industrial productions [7].
Recently,a new process for preparing DEC from urea and ethanol described in Scheme 1 becomes more attractive. The raw materials are low in price and the byproduct ammonia can be used as a recycled material for urea production [8]. This reaction proceeds in two steps: ethyl carbamate (EC) is formed as an intermediate initially and then it can be further converted to DEC. The first step is fast and EC can be easily obtained even without a catalyst. However,the subsequent EC ethanolysis in the second step is difficult to perform [9]. And until now,this process was usually conducted in the presence of catalysts such as organotin compounds,alkali metal compounds and metal oxides [10]. Wang et al. [11] conducted the urea ethanolysis over a series of metal oxides at 463 K in 5 h. The highest yield of DEC 14.2% was achieved using ZnO as a catalyst,while in the absence of catalysts the yield of DEC was 0%. Zhao et al. [12] reported that with PbO as catalyst,the highest yield of DEC was 16.2% with 44.0% selectivity at 463 K in 7 h. Compared to the single metal oxide catalysts,An et al. [13] provided insights into double metal oxide for the reaction of ethyl carbamate and ethanol,a higher yield of DEC of 20.6% was achieved over ZnO-PbO but DEC selectivity was only 41.0% at 463 K in 7 h. So,in previous studies,expensive catalysts were indispensable and the reaction equilibrating time was very long. Accordingly,it is vital to identify new methods to promote the reaction in a more environmentally benign and commercially viable way.

|
Download:
|
| Scheme 1.The reaction equation between urea and ethanol (1),ethyl carbamate and ethanol (2) and the side reaction of NEEC and diethyl carbonate. | |
Supercritical fluids (SCFs) technology has attracted increasing attention in recent years. SCFs have been widely used in organic syntheses [14],biodiesel production [15] and chemical degradation [16]. The superiorities of SCFs are summarized as the follows: (1) They are green and catalyst-free; (2) High yield and selectivity are always achieved by manipulating reaction temperature and pressure of SCFs [17]. Supercritical ethanol is one of the most promising SCFs because of its milder critical conditions (243.1 ℃,6.38 MPa),non-toxicity and high activity. To the best of our knowledge,there have been no report on the synthesis of DEC from EC in supercritical ethanol. Based on the unique properties of supercritical ethanol,we expected its good performance in the ethanolysis reaction. 2. Experimental
Ethyl carbamate (A.R.),ethanol (A.R.),diethyl carbonate (A.R.),acetone (A.R.),dodecane (A.R.) were commercially available from Sinopharm Chemical Reagent and were used as received.
An assembled stainless-steel reactor with a volume of 500 μL served as the batch-type reactor. Before it use,the reactor should be cleaned at 573 K for 8 h with 300 μL of deionized water.
The reaction solution was prepared by dissolving EC in ethanol with the aid of ultrasound at room temperature. An exact amount of reactant was fed to the stainless-steel reactor by a microliter syringe. Reactant volumes at different temperature were as follows: 315 μL at 543 K,305 μL at 553 K,295 μL at 563 K,285 μL at 573 K and 275 μL at 583 K. After loading,the reactor was sealed and immersed into a molten-salt bath (consisted of 40 wt% NaNO2,7 wt% NaNO3 and 53 wt% KNO3) that has been preheated to the required temperature. The temperature was controlled at ±2 K by an Omega temperature controller. After a desired reaction time,the reactor was placed into ice water for about 10 min to quench the reaction. The liquid reactant mixture was taken out by microliter syringe. Then the reactor was treated with acetone for three times to ensure that the reaction mixture was completely transferred.
The liquid reaction contents were analyzed quantitatively by gas chromatograph (SHIMADZU GC-2014) equipped with an RT- 10223 capillary column (30 m ×0.25 mm × 0.25 μm). The analytical conditions were: injector temperature 553 K,column temperature program-raised from 313 K to 523 K at a rate of 15 K/min,nitrogen at a flow rate of 3 mL/min served as the carrier gas. Ndodecane was selected as an internal standard.
The products were identified by GC-MS (Varian Saturn 3900/ 2100) equipped with a VF-5MS non-polar column (30 m ×0.25 mm × 0.25 μm). The analytical conditions were: injector temperature 523 K,column temperature was settled at 333 K for 3 min,then program-raised to 523 K at a rate of 15 K/min,helium gas at a flow rate of 1 mL/min served as the carrier gas. The product DEC was verified by the fragment ion peaks of m/z 18,29,45,63,75,91,118. The fragment peaks of N-EEC were m/z18,28 44,58,72,88,102,117. These mass data matched exactly with the standard chromatograms in mass spectral library.
The yield and selectivity in this work were defined as the follows:

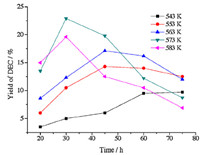
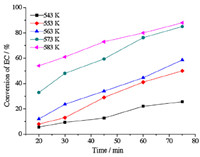
Figs. 1 and 2 depict the effects of reaction temperature (543-583 K) and time (20-75 min) on the conversion of EC and the yield of DEC. It is observed that the conversion of EC and the yield of DEC were both enhanced as the temperature increased from 543 K to 573 K and the yield of DEC reached its maximum of 22.9% with an EC conversion of 48.3% at 573 K. This revealed that synthesis of DEC in supercritical ethanol was greatly affected by temperature. The effect of temperature lied in two aspects: On one hand,as reported in the literature,the reaction was endothermic,so elevated temperatures are favorable according to the laws of thermodynamics [12, 13]; On the other hand,the temperature played a decisive role on the properties of supercritical ethanol [18, 19, 20]. As we know,ethanol under supercritical conditions mainly exists in the form of small oligomers instead of H-bonded aggregates in the liquid. Monomers dominated in these small oligomers and its percentage (ranging from 35.2% to 92.2%) rises as temperature increases. As a result,at higher temperatures,more monomers of ethanol were directly exposed to EC,which increased the chance of collisions of molecules and promoted the reaction. This hypothesis was consistent with our experimental results. However,when the temperature exceeded 573 K,the conversion of EC increased but the yield of DEC decreased. Meanwhile,we also found that the major by-product N-EEC increased significantly,which suggested that at higher temperatures the side reaction described in Eq. (3) by consuming DEC and EC became more significant,which leads to higher conversion of EC and lower yield of DEC. Therefore,the optimum reaction temperature was 573 K for the synthesis of DEC. The effect of temperature on the yield of DEC agreed with the findings in the literature [13].
|
Download:
|
| Fig. 1. The effects of reaction temperature and time on the yield of DEC. The molar ratio of ethanol/EC: 10. | |
|
Download:
|
| Fig. 2. The effects of reaction temperature and time on the conversion of EC. The molar ratio of ethanol/EC: 10. | |
The influence of reaction time on the DEC synthesis was also described in Figs. 1 and 2. The yield of DEC and conversion of EC both increased with increased reaction time at 543 K. At lower temperature,the side reaction can hardly proceed due to the low concentration of DEC,therefore the DEC continually accumulated and the formation of EEC was negligible. Furthermore,at the temperatures ranging from 553 K to 583 K,the conversion of EC increased continuously but the yield of DEC increased initially and then decreased at each temperature. In addition,we found that at longer reaction time,the amount of by-product N-EEC increased significantly,which suggested that the side reaction was accelerated. As a result,the yield and selectivity of DEC were reduced with prolonged reaction time. Therefore,the optimal reaction time was 30 min. The effect of time on the yield of DEC was also in good accordance with the literature [13]. 3.2. Effect of ethanol/EC molar ratio on the yield of DEC
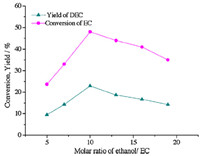
Effect of ethanol/EC molar ratio on the yield of DEC was shown in Fig. 3,as the molar ratio of ethanol/EC varied from 5:1 to 19:1,the yields increased gradually and then declined and the peak was at the ratio of 10:1. As reported in the literature [11],in this reaction,it is difficult for the weaker nucleophile CH3CH2O- to displace the stronger nucleophile NH2-. Therefore,at lower ethanol/EC molar ratio,ethanol is insufficient to promote the reaction thermodynamically. Besides,when the ethanol/EC molar ratio was lower,the amount of by-product N-EEC was higher,which implied that the side reaction of EC N-ethylation took place more easily due to the higher concentration of EC. As a result,the yield of DEC was lower. In contrast,when ethanol/EC molar ratios were too high,the concentration of EC became lower and the reaction was restricted. In conclusion,the optimal ethanol/EC molar ratio was 10:1. The effect of ethanol/EC molar ratio on the yield of DEC was similar to the findings in the literature [13].
|
Download:
|
| Fig. 3. The effects of ethanol/EC molar ratio on the yield of DEC and the conversion of EC. Reaction temperature: 573 K,reaction time: 30 min. | |
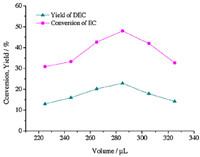
We studied the effect of the reactant loading volume on the yield of DEC by varying the volume from 225 μL to 325 μL in a 500 mL stainless-steel batch type reactor. Reaction conditions are: a temperature of 573 K,a reaction time of 30 min,a molar ratio of 1:10. The reaction pressures at a range of 11.5-14.1 MPa were applied in a 18 mL reaction vessel with an accurate piezometer. As shown in Fig. 4,the yield of DEC increased as the reactant volume increased from 225 to 285 μL and decreased sharply when the volume exceeded 285 μL. The observation can be explained that in the case of lower volume,the number of molecules per unit volume of the reaction vessel was fewer and the reaction rate was lower,so the yield of DEC increased with an increased reactant volume. However,as the reactant volume passed the optimum,the increased pressure resulted in the re-formation of the H-bonded aggregates at the expense of ethanol monomers. Therefore,the optimal reaction conditions were as follows: reactant volume 285 μL,pressure 13.2 MPa,temperature 573 K,reaction time 30 min,a maximum yield of DEC was 22.9% with a selectivity of 48.0%.
|
Download:
|
| Fig. 4. The effects of reactant loading volume on the yield of DEC and the conversion of EC. Reaction temperature: 573 K,reaction time: 30 min,the molar ratio of ethanol/EC: 10,reaction pressure: 11.5 MPa (225 μL),12.1 MPa (245 μL),12.6 MPa (265 μL),13.2 MPa (285 μL),13.6 MPa (305 μL),14.1 MPa (325 μL). | |
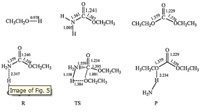
The possible reaction mechanization was obtained on the basis of DFT study preliminarily. As shown in Fig. 5,by geometry optimization of ethyl carbamate and methanol,a weak H-bonded complex (R) formed,originated from which the transition state (TS) was appeared after further optimization. In TS,the carbonyl C and ethoxy O became closer and the H atom in hydroxyl was attached to the amino N atom which resulted in the bond of N-C extended. At the same time,the carbonyl C changes from sp2 hybridization to almost sp3 hybridization and the dihedral angle of TS was 132.8°. Then,the product diethyl carbonate and ammonia formed. The results were accurately consistent with the literature published [21]. The possible reaction mechanization was shown in Scheme 2.
|
Download:
|
| Fig. 5. The optimized geometries for reactant (ethanol and EC),intermediate (M),transition state (TS) and product | |

|
Download:
|
| Scheme 2.Possible reaction mechanism for DEC synthesis from EC in supercritical ethanol. | |
A novel process for the synthesis of DEC from EC was achieved under catalyst-free conditions in supercritical ethanol,in which ethanol act as both solvent and active reactant. The possible reaction mechanism was also proposed preliminarily on the basis of DFT study. Compared with traditional methods,the method presented herein showed many advantages,such as satisfactory yield of 22.9%,high selectivity of 48.0%,remarkably short reaction time and absence of catalysts. In addition,our work brought the synthesis of DEC from EC and ethanol much closer to green industrial development. Accordingly,we expect eagerly that supercritical ethanol can play a more important role in chemical industry in the near future.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (No. 21073064).
| [1] | B.C. Dunn, C. Guenneau, S.A. Hilton, et al., Production of diethyl carbonate from ethanol and carbon monoxide over a heterogeneous catalyst, Energy Fuels 16 (2002) 177-181. |
| [2] | J.X. Zhen, S.Y. Hua, C.S. Hua, Novel synthesis of diethyl carbonate over palladium/ MCM-41 catalysts, Catal. Lett. 69 (2000) 153-156. |
| [3] | T.J. Bruno, A. Wolk, A. Naydich, et al., Composition-explicit distillation curves for mixtures of diesel fuel with dimethyl carbonate and diethyl carbonate, Energy Fuels 23 (2009) 3989-3997. |
| [4] | A.A.G. Shaikh, Organic carbonates, Chem. Rev. 96 (1996) 951-976. |
| [5] | P.B. Zhang, Z. Zhang, S.P. Wang, et al., A new type of catalyst PdCl2/Cu-HMS for synthesis of diethyl carbonate by oxidative carbonylation of ethanol, Catal. Commun. 8 (2007) 21-26. |
| [6] | H. Krimm, H.J. Buysch, H. Rudolph, Process for the preparation of dialkyl carbonates, U.S. Patent 4,307,032, Dec. 22, 1981 |
| [7] | W.B. Zhao, W.C. Peng, D.F. Wang, et al., Zinc oxide as the precursor of homogenous catalyst for synthesis of dialkyl carbonate from urea and alcohols, Catal. Commun. 10 (2009) 655-658. |
| [8] | M.H. Wang, N. Zhao, W. Wei, et al., Synthesis of dimethyl carbonate from urea and methanol over ZnO, Ind. Eng. Chem. Res. 44 (2005) 7596-7599. |
| [9] | J. Sun, B. Yang, H. Lin, A semi-continuous process for the synthesis of methyl carbamate from urea and methanol, Chem. Eng. Technol. 27 (2004) 435-439. |
| [10] | C.C. Wu, X.Q. Zhao, Y.J. Wang, Effect of reduction treatment on catalytic performance of Zn-based catalyst for the alcoholysis of urea to dimethyl carbonate, Catal. Commun. 6 (2005) 694-698. |
| [11] | D.P. Wang, B.L. Wang, X.W. Zhai, et al., Synthesis of diethyl carbonate by catalytic alcoholysis of urea, Fuel Process. Technol. 88 (2007) 807-812. |
| [12] | H.L. Zhao, X.Q. Zhao, H.L. An, et al., Synthesis of diethyl carbonate from ethyl carbamate and ethanol over leadoxide catalyst, Petrochem. Technol. 38 (2009) 139-144. |
| [13] | H.L. An, X.Q. Zhao, L. Guo, et al., Synthesis of diethyl carbonate from ethyl carbamate and ethanol over ZnO-PbO catalyst, Appl. Catal. A. 433/434 (2012) 229-235. |
| [14] | G. Brunner, Applications of supercritical fluids, Annu. Rev. Chem. Biomol. Eng. 1 (2010) 321-342. |
| [15] | M.M. Gui, K.T. Lee, S. Bhatia, Supercritical ethanol technology for the production of biodiesel: process optimization studies, J. Supercrit. Fluids 49 (2009) 286-292. |
| [16] | H. Jie, H. Ke, Z. Qing, et al., Study on depolymerization of polycarbonate in supercritical ethanol, Polym. Degrad. Stab. 91 (2006) 2307e2314. |
| [17] | G. Madras, C. Kolluru, R. Kumar, Synthesis of biodiesel in supercritical fluids, Fuel 83 (2004) 2029-2033. |
| [18] | D. Dellis, M. Chalaris, J. Samios, Pressure and temperature dependence of the hydrogen bonding in supercritical ethanol: a computer simulation study, J. Phys. Chem. B 109 (2005) 18575-18590. |
| [19] | J. Lu, E.C. Boughner, Nearcritical and supercritical ethanol as a benign solvent: polarity and hydrogen-bonding, Fluid Phase Equilib. 198 (2002) 37-49. |
| [20] | P. Lalanne, J.M. Andanson, J.C. Soetens, et al., Hydrogen bonding in supercritical ethanol assessed by infrared and Raman spectroscopies, J. Phys. Chem. A 108 (2004) 3902-3909. |
| [21] | Y.Y. Gao, W.C. Peng, N. Zhao, et al., A DFT study on the reaction mechanism for dimethyl carbonate synthesis from methyl carbamate and methanol, J. Mol. Catal. A: Chem. 351 (2011) 29-40. |




