b Zhejiang Provincial Key Laboratory of Health Risk Appraisal for Trace Toxic Chemicals, Zhejiang University, Hangzhou 310028, China;
c School of Pharmacy, East China University of Science and Technology, Shanghai 200237, China;
d Department of Chemistry, Lishui University, Lishui 323000, China;
e Thermofisher Scientific, Hangzhou 310028, China
Iminodiacetic acid (IDA) is widely used as an intermediate in industry. It has a nitrogen atom and two short chain carboxylic groups,which make it a great chelating agent,for it is able to form a metal complex with two fused five-membered chelate rings [1, 2]. Currently,IDA has been applied in a lot of fields from metal ion removal [3],in vivo imaging [4],chemical separations [5, 6],and stationary phases synthesis [7, 8]. Because of its nitrogen atom and short chain carboxylic groups,IDA is widely used as an intermediate in the manufacture of chelating agents,glyphosate herbicides,and surfactants [9, 10, 11].
Up to now,several methods have been described for the determination of IDA. The common method to determine it is via acid-alkali titration. Other analytical techniques like GC with precolumn derivatization [12] and UV spectrophotometry detecting iron(Ⅲ)-IDA complex [13] have also been studied. However,both of them suffer from tedious sample preparation to convert the analytes into derivatives. Because of the requirement for a better detection limit,liquid chromatographic procedures have been developed. Jennifer et al. proposed a novel method using HPLC with tris(2,2'-bipyridyl) ruthenium(Ⅱ) electrogenerated chemiluminescence detection [14]. Ion chromatography (IC),since its first introduction in the mid-1970s [15],has been a useful tool for detecting ionic substances. Hydrophilic substances can be determined quickly and conveniently using it [16, 17, 18]. IDA is a kind of amino acid that has two carboxylic groups,with pKa1 = 2.98,pKa2 = 9.89,respectively,and it is a hydrophilic substance. So,suppressed conductivity ion chromatography is quite suitable for the detection of IDA.
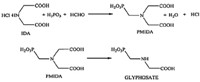
Glyphosate [HO2CCH2NHCH2PO3H2,N-(phosphonomethyl)- glycine] is the active ingredient in several commercial herbicides and widely used in various applications for weed and vegetation control [19, 20]. Many chemical routes for synthesizing glyphosate have been reported,among which the IDA chemical pathway is one of the most effective and used by Monsanto Company,the principle company producing it in the world [11, 21, 22, 23] (Fig. 1). Because of its prominence in agriculture,the need exists to determine glyphosate and its related compounds in several settings,ranging from its quantification during production to the detection of trace impurities in it. The main objective of this work was to develop a simple and sensitive method for the determination of IDA in glyphosate. The determination of the component was based on IC with suppressed conductivity detection.
|
Download:
|
| Fig. 1. Synthesis of glyphosate via IDA chemical pathway. | |
The ion chromatographic analysis was performed on an ICS-2000 (ThermoFisher Scientific,Sunnyvale,CA,USA) equipped with an isocratic pump,a six-port valve fitted with a 25 μL sample loop,a DS6 heated conductivity detector,a column heater,and an EGCKOH eluent generator. Data were acquired by using Chromeleon 6.8 software (ThermoFisher Scientific,USA). Eluent flow rate was set at 1 mL/min. For the analytical separation,an IonPac AS11-HC (50 mm × 4 mm) guard column and an IonPac AS11-HC (250 mm × 4 mm) analytical column were used. In addition,a Thermo ASRS-4 mm suppressor was utilized in the recycle mode. Polyether ether ketone (PEEK) tubes with the lengths as short as possible were used to connect all chromatographic hardware. A JY92-π sonifier cell disrupter (Scientz Biotechnology Co.,Ltd.,Ningbo,China) was employed to extract IDA in samples. 2.2. Reagents and samples
All reagents were of analytical reagent grade. Iminodiacetic acid (IDA) was obtained from Huipu Chemical Reagent Co.,Ltd. (Zhejiang,China). A water purification system (Millipore,Milford,MA,USA) was used to further deionize distilled water for all solutions and eluents. Standard solutions of F-,Cl-,Br-,NO2 -,NO3 - and SO42- were prepared by appropriate dilution of their anion standard stock NCS (NCS analytical instruments Co.,Ltd.,Beijing,China) solution of analytical grade (1000 mg/L) for IC to obtain the desired concentrations of each analyte. All solutions were stored in tightly closed containers at 4 ℃. The glyphosate samples were all purchased from the market made by three different companies. All solutions and samples were stored in tightly closed containers at 4 ℃. 2.3. Sample preparation
Approximately 100 mg of glyphosate sample was weighed into a 100 mL volumetric flask then diluted to volume with deionized water. Sample solutions were filtered through a 0.22 μm membrane filter before sample injection. 2.4. Chromatographic conditions
The optimized eluent solution contained 15 mmol/L KOH generated by an EGC-KOH eluent generator from 0 to 12.5 min to analyze IDA and other anions with weak retention,while anions with strong retention,such as glyphosate,were washed down by 40 mmol/L KOH later. Separation was carried out under isocratic conditions at a flow-rate of 1 mL/min and a constant temperature of 35 ℃. Sample preparation: Approximately 100 mg of glyphosate sample was weighed into a 100 mL volumetric flask then diluted to volume with deionized water. Sample solutions were filtered through a 0.22 mm membrane filter before sample injection. 3. Results and discussion 3.1. Method development
The use of the ICS-2000 system allowed higher detection sensitivity because of the low background conductivity.
Due to the weak retention of IDA and also the routine analysis of the conventional inorganic anions,columns with a high anionexchange capacity were appropriate in this system. An Ion Pac AS11-HC high capacity anion separation column with a capacity of 290 μequiv./column coupled with an Ion Pac AS11-HC guard column with a capacity of 7 mequiv./column was selected for this system.
The eluent is important for analysis of the liquid chromatography data. Different concentrations of KOH (5,15,20,30 mmol/L) have been studied to determine the optimum concentration with an injection volume of 25 μL and suppressed conductivity detection. The results showed that the separation of IDA with other conventional anions was not good at the higher concentrations,and the lower concentrations resulted in a broad peak shape. It is better to separate these anions with 15 mmol/L KOH as the optimum concentration of eluent. For the purpose of washing the anions with long retention times down from the column,higher concentrations of KOH (30,40,50 mmol/L) have been studied. 40 mmol/L KOH was selected as it can wash down anions as well as the 50 mmol/L,yet it is more economic and faster than the 30 mmol/L.
As a consequence,we have chosen 15 mmol/L KOH as the eluent from 0 to 12.5 min,and 40 mmol/L from 12.6 to 25 min with a flow rate of 1 mL/min. 3.2. Method validation
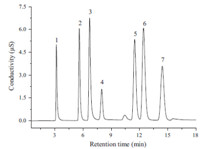
Under the optimized conditions above,a series of standard solutions consisting of IDA and conventional inorganic anions were analyzed. A representative chromatogram of a standard solution is shown in Fig. 2. The precision of the method was evaluated by making repeated analyses of a fixed concentration. The relative standard deviations (RSDs) for the retention times and peak areas for six analyses within one day were less than 0.66% and 1.53%,respectively. Inter-day RSDs of retention times and peak areas (5 days) were less than 1.39% and 2.75%,respectively. The calibration curve was evaluated by plotting peak areas against the concentrations of the anion. The linear calibration curve was obtained in the corresponding concentration range,from 0.1 mg/L to 25 mg/L. The correlation coefficient for IDA was 0.9997,showing good linearity. The limit of detection (LOD) of IDA,based on S/N = 3 and a 25 μL injection volume,was 31.8 μg/L. The value was low enough for the analysis of IDA in glyphosate. Compared to the previously published method,the detection limit of IDA is lower due to the good detectability of ion chromatography combined with ECD.
|
Download:
|
| Fig. 2. Standard chromatogram of IDA and conventional inorganic anions. Eluent: 15 mmol/L KOH (0-12.5 min) and 40 mmol/L KOH (after 12.5 min) generated by an EGC-KOH eluent generator; flow rate: 1.0 mL/min; injection volume: 25 mL; conductivity detector. Peaks: (1) fluoride,1 mg/L; (2) chloride,2.5 mg/L; (3) nitrite,5 mg/L; (4) IDA,8 mg/L; (5) sulfate,5 mg/L; (6) bromide,10 mg/L; (7) nitrate,10 mg/L. | |
IDA was determined in three real samples using the proposed method. The sample weight of about 0.10 g was diluted to 100 mL. The chromatogram corresponding to one of the representative samples is shown in Fig. 3. With quantitative use of the standard curve method,the content of IDA in one of the samples was 1.980 g/kg,while it was not detected in the other two samples. Recovery studies were tested by the standard addition method,and the obtained values ranged between 92.8% and 103.6%. The results indicate this method has the advantages of high accuracy and can meet the requirements for quantitative analysis of IDA.

|
Download:
|
| Fig. 3. Chromatogram of glyphosate sample. Eluent: 15 mmol/L KOH (0-12.5 min) and 40 mmol/L KOH (after 12.5 min) generated by an EGC-KOH eluent generator; injection volume: 25 mL; flow rate: 1.0 mL/min; conductivity detector. Peaks: (1) chloride; (2) IDA; (3) glyphosate. | |
The method provides advantages in the determination of IDA in glyphosate. The RSDs showed that the stability and matrix spike recoveries were quite satisfactory. IDA content in commercial glyphosate products were analyzed by means of ion chromatography using a gradient elution with KOH as the mobile phase and conductor detection.
Acknowledgments
This research was financially supported by the National Important Project on Science Instrument (No.2012YQ09022903),Zhejiang Provincial Natural Science Foundation of China (No.Y4110532),and Zhejiang Provincial Assay Foundation of China (No.2012C37038).
| [1] | S. Chaberek Jr., A.E. Martell, Stability of metal chelates. I. Iminodiacetic and iminodipropionic acids, J. Am. Chem. Soc. 74 (1952) 5052-5056. |
| [2] | R.C. Courtney, R.L. Gustafson, S. Chaberek Jr., A.E. Martell, Hydrolytic tendencies of metal chelate compounds. II. Effect of metal ion, J. Am. Chem. Soc. 80 (1958) 2121-2128. |
| [3] | C.C. Wang, C.Y. Chen, C.Y. Chang, Synthesis of chelating resins with iminodiacetic acid and its wastewater treatment application, J. Appl. Polym. Sci. 84 (2002) 1353-1362. |
| [4] | K.M. Harmatys, E.L. Cole, B.D. Smith, In vivo imaging of bone using a deep-red fluorescent molecular probe bearing multiple iminodiacetate groups, Mol. Pharmaceutics 10 (2013) 4263-4271. |
| [5] | A. Yuchi, T. Sato, Y. Morimoto, H. Mizuno, H. Wada, Adsorption mechanism of trivalent metal ions on chelating resins containing iminodiacetic acid groups with reference to selectivity, Anal. Chem. 69 (1997) 2941-2944. |
| [6] | Z. Rassi, C. Horvaá th, Metal chelate-interaction chromatography of proteins with iminodiacetic acid-bonded stationary phases on silica support, J. Chromatogr. A 359 (1986) 241-253. |
| [7] | E. Sugrue, P. Nesterenko, B. Paull, Ion exchange properties of monolithic and particle type iminodiacetic acid modified silica, J. Sep. Sci. 27 (2004) 921-930. |
| [8] | F. Alvaro, C. Claudio, F. Binyamin, L.K. Barry, High-performance immobilizedmetal affinity chromatography of proteins of iminodiacetic acid silica-based bonded phases, J. Chromatogr. A 371 (1986) 335-352. |
| [9] | Z.Q. Liu, F.F. Li, F. Cheng, et al., A novel synthesis of iminodiacetic acid: biocatalysis by whole alcaligenes faecalis ZJB-09133 cells from iminodiacetonitrile, Biotechnol. Prog. 27 (2011) 698-705. |
| [10] | L.B. Ni, R.H. Zhang, Q.X. Liu, et al., pH-and mol-ratio dependent formation of zinc(Ⅱ) coordination polymers with iminodiacetic acid: synthesis, spectroscopic, crystal structure and thermal studies, J. Solid State Chem. 182 (2009) 2698-2706. |
| [11] | A.T. Woodburn, Glyphosate: production, pricing and use worldwide, Pest Manag. Sci. 56 (2000) 309-312. |
| [12] | C.B. Warren, E.J. Malec, Quantitative determination of nitrilotriacetic acid and related aminopolycarboxylic acids in inland waters: analysis by gas chromatography, J. Chromatogr. 64 (1972) 219-237. |
| [13] | S.N. Bhattacharyya, N.C. Saha, Spectrophotometric determination of iminodiacetic acid in presence of primary amino-acids, Talanta 23 (1976) 331-332. |
| [14] | J.S. Ridlen, G.J. Klopf, T.A. Nieman, Determination of glyphosate and related compounds using HPLC with tris(2,2'-bipyridyl)ruthenium(Ⅱ) electrogenerated chemiluminescence detection, Anal. Chim. Acta 341 (1997) 195-204. |
| [15] | H. Small, T.S. Stevens, W.S. Bauman, Novel ion exchange chromatographic method using conductimetric detection, Anal. Chem. 47 (1975) 1801-1809. |
| [16] | P.E. Jackson, C.A. Pohl, Advances in stationary phase development in suppressed ion chromatography, Trends Anal. Chem. 16 (1997) 393-400. |
| [17] | R. Saari-Nordhaus, L. Nair, J.M. Anderson, Dual-column techniques for the simultaneous analysis of anions and cations, J. Chromatogr. A 602 (1992) 127-133. |
| [18] | D. Yan, G. Schwedt, Simultaneous ion chromatography of inorganic anions together with some organic anions and alkaline earth metal cations using chelating agents as eluents, J. Chromatogr. A 516 (1990) 383-393. |
| [19] | Y. Zhu, F.F. Zhang, C.L. Tong, W.P. Liu, Determination of glyphosate by ion chromatography, J. Chromatogr. A 850 (1999) 297-301. |
| [20] | W.A. Battaglin, M.T. Meyer, K.M. Kuivila, J.E. Dietze, Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, ground water, and precipitation, J. Am. Water Resour. Assoc. 50 (2014) 275-290. |
| [21] | L.J. Marek, W.C. Koskinen, Simplified analysis of glyphosate and aminomethyl-phosphonic acid in water, vegetation and soil by liquid chromatography-tandem mass spectrometry, Pest Manag. Sci. 70 (2014) 1158-1164. |
| [22] | G.M. Xu, L. Zhou, D.Y. Zheng, et al., Process for preparation of glyphosate by iminodiacetic acid, Agrochemicals 46 (2007) 656-658. |
| [23] | G.M. Dill, R.D. Sammons, P.C.C. Feng, et al., Glyphosate: Discovery Development, Applications, and Properties, in: V.K. Nandula (Ed.), Glyphosate Resistance in Crops and Weeds: History, Development, and Management, Wiley, Hoboken, New Jersey, 2010, pp. 19-20. |




