b Department of Neuroscience, University of Wisconsin Medical School, Madison WI 53706-1532, USA
Diaryl sulfides constitute an important class of therapeutic agents in medicinal chemistry [1,2,3,4,5]. Therefore,the development of efficient and mild methods for the synthesis of these compounds represents a broad area of organic chemistry. In recent years,C-S cross-coupling reaction has drawn high attention due to their promise in numerous bioactive natural products and pharmaceuticals.
Traditional methods for the synthesis of diaryl/aryl alkyl sulfides involve reduction of aryl sulfones or aryl sulfoxides using strong reducing agents [6]. To overcome these harsh conditions, transition-metal compounds via catalyzed cross-coupling systems, such as nickel [7,8,9,10],palladium [11,12],lanthanum [13],indium [14],have been developed.
Some of the methods for the C-S bond formation using crosscoupling systems involves reaction of aryl diazoniumfluoroborate and diaryl disulfides [15],coupling of aryl or alkyl thiols with aryl halides [8,16,17,18,19,20],cross-coupling reaction of aryl halides and ethyl potassium xanthogenate [21,22],reaction of phenolic esters such as acetates,triflates,tosylates and phosphonates with alkyl halides by using thiourea as a sulfur source [23],reaction of aryl halides with aminothiourea [24],oxidative sulfidation of diaryl disulfides with aryltrimethoxysilanes [25],reaction of thiourea with aryl halides [26].
Previous approaches to the synthesis of diaryl/aryl alkyl sulfides usually require more drastic conditions,such as high reaction temperature,use of stoichiometric amounts of the catalyst,use of ligands together with highly toxic metals. Also,it must be noted that all of the previous methods suffer from the lack of recyclability,which represent more obstacles for the utility of this chemistry in parallel synthesis.
Heterogeneous catalysis is of paramount importance in many are as [27,28,29,30,31,32]. Heterogeneously catalyzed processes have recently been brought to many scientists as comparatively more efficient methodologies because facile recovery and reusability of the catalysts can be done with these processes. Also stability of the catalyst and even sometimes its activity will be improved when heterogeneously a catalytic system is used [33,34]. Among these heterogeneous catalysts,magnetic nanoparticles have emerged as an attractive method due to their numerous applications in organic synthesis [35,36,37,38,39,40,41,42,43,44]. Copper/iron oxide based catalysts are environmentally compatible,air and moisture insensitive,and separation from reaction mixture is very simple by means of an external magnetic field [45,46,47].
Only two new reports of magnetically separable CuFe2O4 as a catalyst in synthesis of diaryl chalcogenides has been published in the literature. The first report on magnetically separable,recyclable nano-CuFe2O4 catalyzed reaction of aryl/alkyl iodides with aryl/alkyl thiols has been published by Nageswar and co-workers in 2011 [48]. Two years later,organoselenides and organotellurides have been synthesized by a simple reaction of organoboronic acids and dichalcogenides using CuFe2O4 nanoparticles without any ligand [49]. These two works encouraged us to use copper/iron oxide based nanoparticles for S-arylation reaction of thiourea by aryl halides.
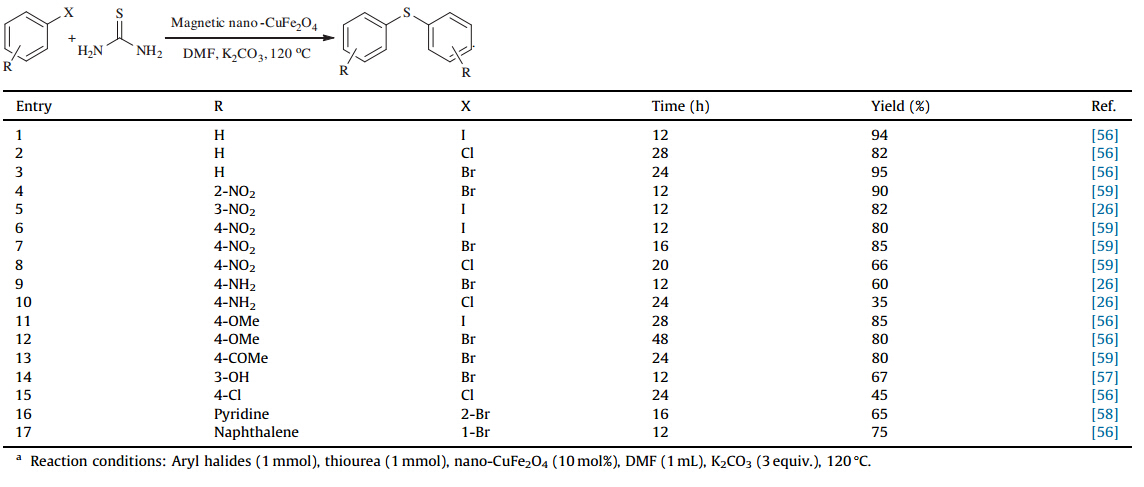
On the direct synthesis of diaryl sulfides from the reaction of thiourea and aryl halides,few reports have been published in literatures [23,26,50,51,52,53,54]. In this paper,we describe a methodology for the synthesis of these symmetrical sulfides using a ligand-free and high-recyclable magnetic catalyst. This methodology gives access to a wide variety of symmetrical aryl sulfide derivatives under milder conditions (Scheme 1).

|
Download:
|
| Scheme 1.S-Arylation of thiourea by aryl halides in the presence of nano-CuFe2O4. | |
Nano-CuFe2O4 was prepared with a modified method according to the literature [55]. Both 1.51 g Cu(NO3)2.3H2O and 5.04 g Fe(NO3)3.9H2O were dissolved in 100 mL distilled water,then added to 50 mL of 4 mol/L NaOH solution followed by heating and aging at 90 ℃ for 2 h. The prepared nanoparticles were centrifuged, washed by water and dried at 80 ℃ overnight. Finally the powder was calcined at 800 ℃ for 2 h. X-ray diffraction,FT-IR,SEM and EDX analyses confirmed the formation of the nano-CuFe2O4(Figs. S1-S6 in Supporting information). The average particles size was 23.5 nm by the Scherrer equation. 2.2. General procedure for the synthesis of diphenyl sulfane
In a typical experiment,a flask equipped with a magnetic stirrer was charged with 0.2 mmol base,0.25 mmol thiourea (1 equiv.), 0.5 mmol iodobenzene (1 equiv.),1 mL DMF and 10 mol% nano-CuFe2O4 catalyst. This mixture was heated at 120 ℃ in an oil bath for 12 h. The progress of the reaction was monitored by TLC (n-hexane). Then the reaction mixture was cooled to room temperature,and the catalyst was separated magnetically. The supernatant was extracted with 10 mL n-hexane (for five times). The organic layer was dried with CaCl2 and then the solvent removed under reduced-pressure to give the product. In some cases,derivatives of diphenyl sulfide were purified by column chromatography on silica gel (with n-hexane) to give the pure diaryl sulfide. All products are known compounds and were identified by comparison of their properties (1H NMR and 13C NMR) with those of authentic samples [26,56,57,58,59,60]; these data were given in the Supporting information. 3. Results and discussion
To optimize reaction conditions,we undertook an intensive screening of reaction variables using iodobenzene as representative substrate (Table 1). These studies were carried out using a variety of different bases (Na2CO3,K2CO3,Cs2CO3,NaOH and KOH). Among the studied bases,Cs2CO3 and K2CO3 were the best (Table 1, entries 1 and 8). Replacing Cs2CO3 by the less expensive K2CO3 had no deleterious effect on the reaction. Yields of the product increased with increasing amounts of the base,so,three equivalents of K2CO3 were chosen as a standard amount of base for this reaction (Table 1,entry 13). Adjusting the reaction mixture showed that no product was formed in the absence of the base (Table 1,entry 15). Optimization with respect to the solvent showed that the reaction proceeds best in DMF (Table 1,entry 1). This solvent gave better results than other solvents,such as NMP, DMSO,CH3CN (Table 1,entries 2-4). No product has been observed in the presence of water as a reaction medium (Table 1,entry 5). Screening was also performed at different temperatures and results showed that no reaction occurred below 100 ℃ (Table 1, entries 17 and 18). Ultimately,the optimal temperature was determined to be 120 ℃,and this was then used as a standard for the optimization of other parameters. The ratio of thiourea to aryl iodide was also optimized. An excess of thiourea is generally required to achieve the best coupling results. In our experiments,it was shown that a 100% excess of thiourea (2 equiv.) provided full conversion to the product in 24 h (Table 1,entry 16) when monitored by GC. Finally,the amounts of the catalyst were also examined in the range of 1-30 mol% of the nano-CuFe2O4 and it was found that 10 mol%,and in some cases 20 mol%,gave the most consistent results (Table 1,entries 13,14 and 16). Without nano-CuFe2O4,no product was observed and even running the reaction in the presence of low amounts of the catalyst (1-2 mol%) did not meet with success (Table 1,entries 10 and 11). To probe more fully the role of the copper in the reaction,we ran the coupling reaction in the presence of nano-CoFe2O4 as a source of cobalt and failed to obtain any product. This,therefore,suggests that copper is essential to the success of the reaction.
| Table 1 Optimization of the nano-CuFe2O4catalyzed coupling of iodobenzene with thiourea under various conditions.a |
We next decided to investigate the scope of the reaction via this modified procedure. The S-arylation reaction of thiourea was performed with a wide variety of aryl and alkyl halides (Table 2). Among all of the aryl halides studied by this reaction,both electron-donating substituents and electron-withdrawing substituents are found to be compatible. The reaction was found not to be sensitive to steric hindrance by a substituent ortho to the halide (Table 2,entry 4). Unfortunately,alkyl halides were unreactive under these conditions. We also investigated S-arylation of 2-bromopyridine as a heterocyclic compound due to their importance and found that this substrate successfully thio-etherified and afforded the corresponding symmetrical sulfides in good yields.
| Table 2 Scope of the reaction of aryl halides and thiourea,catalyzed by CuFe2O4nanoparticles.a |
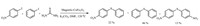
The same protocol was also used in the presence of two different aryl halides to investigate a comparative study. Iodobenzene and 4-iodotoluene were used as substrates and finally both symmetrical and unsymmetrical aryl halides were synthesized (Scheme 2).
|
Download:
|
| Scheme 2.Study of reactivity of S-arylation of thiourea with two different aryl halides. | |
A comparison of the catalytic efficiency of CuFe2O4 with selected previously known catalysts is collected in Table 3. Diphenyl sulfane was produced in 12 h when our catalytic system was used,whereas when nano-CuO was used as catalyst,more reaction time was needed to produce diphenyl sulfane in good yields. Also,it should be mentioned that K2CO3 is preferred over Cs2CO3 due to the low cost of K2CO3,so the less expensive base was used in this work when it is compared with two other reported heterogeneous catalysts as shown in Table 3. Application of the cheaper base and shorter reaction time,along with the ability to separate the catalyst with an external magnet,make our conditions more suitable to other reported works [26,54].
| Table 3 Comparison of protocols for the S-arylation of thiourea by iodobenzene. |
Finally,in order to examine the recyclability of the catalyst, spent CuFe2O4 was easily recovered from the reaction media and re-used. For recycling,after the first use,the catalyst was separated from the reaction mixture by a magnet,washed with ethanol successively,then with water and finally dried at 110 ℃ for 2 h. The regenerated CuFe2O4 was used for the S-arylation of thiourea by iodobenzene under the optimum reaction conditions to study the performance of the re-used catalyst (Table 4). No significant changes in yield of diphenylsulfane were observed after regeneration until the sixth cycle.
| Table 4 The catalyst re-used under the optimum reaction conditions for acetylation of phenol.a |
In summary,we reported on the synthesis of symmetrical aryl/ heteroaryl sulfides from electron-rich and electron-deficient aryl halides without the need for the addition of any ligand. Our methodology involves the use of a non-toxic and magnetic recyclable catalyst. This catalytic system eliminates the need for an inert atmosphere and expensive catalysts. Acknowledgments
We gratefully acknowledge the funding support received for this project from the Isfahan University of Technology (IUT),IR Iran (A.R.H.) and Grant GM 33138 (A.E.R.) from the National Institutes of Health,USA. Further financial support from the Center of Excellency in Chemistry Research (IUT) is gratefully acknowledged. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.015.
| [1] | G. de Martino, M.C. Edler, G. La Regina, et al., New arylthioindoles: potent inhibitors of tubulin polymerization. 2. Structure-activity relationships and molecular modeling studies, J. Med. Chem. 49 (2006) 947-954. |
| [2] | A. Gangjee, Y. Zeng, T. Talreja, et al., Design and synthesis of classical and nonclassical 6-arylthio-2,4-diamino-5-ethylpyrrolo[2,3-d]pyrimidines as antifolates, J. Med. Chem. 50 (2007) 3046-3053. |
| [3] | S.F. Nielsen, E.Ø. Nielsen, G.M. Olsen, T. Liljefors, D. Peters, Novel potent ligands for the central nicotinic acetylcholine receptor: synthesis, receptor binding, and 3DQSAR analysis, J. Med. Chem. 43 (2000) 2217-2226. |
| [4] | S.W. Kaldor, V.J. Kalish, J.F. Davies, et al., Viracept (Nelfinavir Mesylate, AG1343): a potent, orally bioavailable inhibitor of HIV-1 protease, J. Med. Chem. 40 (1997) 3979-3985. |
| [5] | S.V. Ley, A.W. Thomas, Modern synthetic methods for copper-mediated C(aryl)-O, C(aryl)-N, and C(aryl)-S bond formation, Angew. Chem. Int. Ed. 42 (2003) 5400-5449. |
| [6] | H. Firouzabadi, A. Jamalian, Reduction of oxygenated organosulfur compounds, J. Sulfur Chem. 29 (2008) 53-97. |
| [7] | Y. Yatsumonji, O. Okada, A. Tsubouchi, T. Takeda, Stereo-recognizing transformation of (E)-alkenyl halides into sulfides catalyzed by nickel(0) triethyl phosphite complex, Tetrahedron 62 (2006) 9981-9987. |
| [8] | X.B. Xu, J. Liu, J.J. Zhang, Y.W. Wang, Y. Peng, Nickel-mediated inter-and intramolecular C-S coupling of thiols and thioacetates with aryl iodides at room temperature, Org. Lett. 15 (2013) 550-553. |
| [9] | P. Guan, C. Cao, Y. Liu, et al., Efficient nickel/N-heterocyclic carbene catalyzed C-S cross-coupling, Tetrahedron Lett. 53 (2012) 5987-5992. |
| [10] | J. She, Z. Jiang, Y.G. Wang, Simple, efficient and recyclable catalytic system for performing copper-catalyzed C-S coupling of thiols with aryl iodides in PEG and PEG-H2O, Tetrahedron Lett. 50 (2009) 593-596. |
| [11] | Z. Jiang, J. She, X.F. Lin, Palladium on charcoal as a recyclable catalyst for C-S crosscoupling of thiols with aryl halides under ligand-free conditions, Adv. Synth. Catal. 351 (2009) 2558-2562. |
| [12] | N. Park, K. Park, M. Jang, S. Lee, One-pot synthesis of symmetrical and unsymmetrical aryl sulfides by Pd-catalyzed couplings of aryl halides and thioacetates, J. Org. Chem. 76 (2011) 4371-4378. |
| [13] | V.P. Reddy, K. Swapna, A.V. Kumar, K.R. Rao, Lanthanum-catalyzed stereoselective synthesis of vinyl sulfides and selenides, Tetrahedron Lett. 51 (2010) 293-296. |
| [14] | V.P. Reddy, K. Swapna, A.V. Kumar, K.R. Rao, Indium-catalyzed C-S cross-coupling of aryl halides with thiols, J. Org. Chem. 74 (2009) 3189-3191. |
| [15] | D. Kundu, S. Ahammed, B.C. Ranu, Microwave-assisted reaction of aryl diazonium fluoroborate and diaryl dichalcogenides in dimethyl carbonate: a general procedure for the synthesis of unsymmetrical diaryl chalcogenides, Green Chem. 14 (2012) 2024-2030. |
| [16] | M.T. Lan, W.Y. Wu, S.H. Huang, K.L. Luo, F.Y. Tsai, Reusable and efficient CoCl2 6H2O/cationic 2,20-bipyridyl system-catalyzed S-arylation of aryl halides with thiols in water under air, RSC Adv. 1 (2011) 1751-1755. |
| [17] | H.J. Xu, Y.F. Liang, X.F. Zhou, Y.S. Feng, Efficient recyclable CuI-nanoparticlecatalyzed S-arylation of thiols with aryl halides on water under mild conditions, Org. Biomol. Chem. 10 (2012) 2562-2568. |
| [18] | Y.C. Wong, T.T. Jayanth, C.H. Cheng, Cobalt-catalyzed aryl-sulfur bond formation, Org. Lett. 8 (2006) 5613-5616. |
| [19] | M. Arisawa, T. Suzuki, T. Ishikawa, M. Yamaguchi, Rhodium-catalyzed substitution reaction of aryl fluorides with disulfides: P-orientation in the polyarylthiolation of polyfluorobenzenes, J. Am. Chem. Soc. 130 (2008) 12214-12215. |
| [20] | T. Itoh, T. Mase, A general palladium-catalyzed coupling of aryl bromides/triflates and thiols, Org. Lett. 6 (2004) 4587-4590. |
| [21] | V.K. Akkilagunta, R.R. Kakulapati, Synthesis of unsymmetrical sulfides using ethyl potassium xanthogenate and recyclable copper catalyst under ligand-free conditions, J. Org. Chem. 76 (2011) 6819-6824. |
| [22] | D.J.C. Prasad, G. Sekar, Cu-catalyzed one-pot synthesis of unsymmetrical diaryl thioethers by coupling of aryl halides using a thiol precursor, Org. Lett. 13 (2011) 1008-1011. |
| [23] | H. Firouzabadi, N. Iranpoor, M. Gholinejad, A. Samadi, Copper(I) iodide catalyzes odorless thioarylation of phenolic esters with alkyl derivatives using thiourea in wet polyethylene glycol (PEG 200), J. Mol. Catal. A: Chem. 377 (2013) 190-196. |
| [24] | X.M. Wu, W.Y. Hu, Direct synthesis of diaryl sulfides by copper-catalyzed coupling of aryl halides with aminothiourea, Chin. Chem. Lett. 23 (2012) 391-394. |
| [25] | P.S. Luo, M. Yu, R.Y. Tang, P. Zhong, J.H. Li, Solvent-free copper-catalyzed oxidative S-arylation of 1,2-diaryldisulfides with aryltrimethoxysilane, Tetrahedron Lett. 50 (2009) 1066-1070. |
| [26] | K.H.V. Reddy, V.P. Reddy, J. Shankar, et al., Copper oxide nanoparticles catalyzed synthesis of aryl sulfides via cascade reaction of aryl halides with thiourea, Tetrahedron Lett. 52 (2011) 2679-2682. |
| [27] | D.S. Su, J. Zhang, B. Frank, et al, Metal-free heterogeneous catalysis for sustainable chemistry, ChemSusChem 3 (2010) 169-180. |
| [28] | C. Copéret, M. Chabanas, R. Petroff Saint-Arroman, J.M. Basset, Surface organometallic chemistry: homogeneous and heterogeneous catalysis: bridging the gap through surface organometallic chemistry, Angew. Chem. Int. Ed. 42 (2003) 156-181. |
| [29] | D. Rosenthal, Functional surfaces in heterogeneous catalysis: a short review, Phys. Status Solidi A 208 (2011) 1217-1222. |
| [30] | H. Wang, Z. Liu, Progress in combinatorial heterogeneous catalysis, Prog. Chem. 15 (2003) 256-263. |
| [31] | Y.P. Zhang, A.H. Shi, Y.S. Yang, C.L. Li, Impregnated copper on magnetite as catalyst for the O-arylation of phenols with aryl halides, Chin. Chem. Lett. 25 (2014) 141-145. |
| [32] | A. Rostami, B. Tahmasbi, H. Gholami, H. Taymorian, Supported N-propylsulfamic acid on magnetic nanoparticles used as recoverable and recyclable catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones in water, Chin. Chem. Lett. 24 (2013) 211-214. |
| [33] | T. Bligaard, J.K. Nørskov, Heterogeneous Catalysis in Chemical Bonding at Surfaces and Interfaces, Elsevier, Amsterdam, 2008, pp. 255-321. |
| [34] | A.R. Hajipour, H. Karimi, Synthesis and characterization of hexagonal zirconium phosphate nanoparticles, Mater. Lett. 116 (2014) 356-358. |
| [35] | S. Shylesh, V. Schünemann, W.R. Thiel, Magnetically separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis, Angew. Chem. Int. Ed. 49 (2010) 3428-3459. |
| [36] | D. Astruc, F. Lu, J.R. Aranzaes, Nanoparticles as recyclable catalysts: the Frontier between homogeneous and heterogeneous catalysis, Angew. Chem. Int. Ed. 44 (2005) 7852-7872. |
| [37] | M.B. Gawande, P.S. Branco, R.S. Varma, Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies, Chem. Soc. Rev. 42 (2013) 3371-3393. |
| [38] | S.M. Baghbanian, M. Farhang, CuFe2O4 nanoparticles: a magnetically recoverable and reusable catalyst for the synthesis of coumarins via Pechmann reaction in water, Syn. Commun. 44 (2013) 697-706. |
| [39] | B. Karami, S.J. Hoseini, S. Nikoseresht, S. Khodabakhshi, Fe3O4 nanoparticles: a powerful and magnetically recoverable catalyst for the synthesis of novel calix[4]resorcinarenes, Chin. Chem. Lett. 23 (2012) 173-176. |
| [40] | D. Kundu, T. Chatterjee, B.C. Ranu, Magnetically separable CuFe2O4 nanoparticles catalyzed ligand-free C-S coupling in water: access to (E)-and (Z)-styrenyl-, heteroaryl and sterically hindered aryl sulfides, Adv. Synth. Catal. 355 (2013) 2285-2296. |
| [41] | F. Nemati, R. Saeedirad, Nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups as a magnetically separable catalyst for green and efficient synthesis of functionalized pyrimido[4,5-b]quinolines and indeno fused pyrido[2,3-d]pyr-imidines in water, Chin. Chem. Lett. 24 (2013) 370-372. |
| [42] | F.P. Ma, P.H. Li, B.L. Li, et al., A recyclable magnetic nanoparticles supported antimony catalyst for the synthesis of N-substituted pyrroles in water, Appl. Catal. A: Gen. 457 (2013) 34-41. |
| [43] | P.H. Li, B.L. Li, Z.M. An, et al., Magnetic nanoparticles (CoFe2O4)-supported phosphomolybdate as an efficient, green, recyclable catalyst for synthesis of b-hydroxy hydroperoxides, Adv. Synth. Catal. 355 (2013) 2952-2959. |
| [44] | C. Feng, H.Y. Zhang, N.Z. Shang, S.T. Gao, C. Wang, Magnetic graphene nanocomposite as an efficient catalyst for hydrogenation of nitroarenes, Chin. Chem. Lett. 24 (2013) 539-541. |
| [45] | H. Jiao, G.S. Jiao, J.L. Wang, Preparation and magnetic properties of CuFe2O4 nanoparticles, Syn. React. Inorg. Met. 43 (2013) 131-134. |
| [46] | R. Parella, Naveen, A. Kumar, S.A. Babu, Catalytic Friedel-Crafts acylation: magnetic nanopowder CuFe2O4 as an efficient and magnetically separable catalyst, Tetrahedron Lett. 54 (2013) 1738-1742. |
| [47] | Z.P. Sun, L. Liu, D.Z. Jia, W.Y. Pan, Simple synthesis of CuFe2O4 nanoparticles as gas-sensing materials, Sens. Actuators B: Chem. 125 (2007) 144-148. |
| [48] | K. Swapna, S.N. Murthy, M.T. Jyothi, Y.V.D. Nageswar, Nano-CuFe2O4 as a magnetically separable and reusable catalyst for the synthesis of diaryl/aryl alkyl sulfides via cross-coupling process under ligand-free conditions, Org. Biomol. Chem. 9 (2011) 5989-5996. |
| [49] | D. Kundu, N. Mukherjee, B.C. Ranu, A general and green procedure for the synthesis of organochalcogenides by CuFe2O4 nanoparticle catalysed coupling of organoboronic acids and dichalcogenides in PEG-400, RSC Adv. 3 (2013) 117-125. |
| [50] | H. Firouzabadi, N. Iranpoor, M. Gholinejad, One-pot thioetherification of aryl halides using thiourea and alkyl bromides catalyzed by copper(I) iodide free from foul-smelling thiols in wet polyethylene glycol (PEG 200), Adv. Synth. Catal. 352 (2010) 119-124. |
| [51] | J. Mondal, A. Modak, A. Dutta, et al., One-pot thioetherification of aryl halides with thiourea and benzyl bromide in water catalyzed by Cu-grafted furfural iminefunctionalized mesoporous SBA-15, Chem. Commun. 48 (2012) 8000-8002. |
| [52] | M. Soleiman-Beigi, M. Alikarami, F. Mohammadi, A. Izadi, CuI-catalyzed, symmetrical diaryl sulfides synthesis from aryl halides in the presence of KF/Al2O3: using thiourea and thiosemicarbazide as sulfur donor sources, Lett. Org. Chem. 10 (2013) 622-625. |
| [53] | H. Firouzabadi, N. Iranpoor, M. Abbasi, A facile generation of C-S bonds via onepot, odourless and efficient thia-Michael addition reactions using alkyl, aryl or allyl halides, thiourea and electron-deficient alkenes in wet polyethylene glycol (PEG 200) under mild reaction conditions, Tetrahedron 65 (2009) 5293-5301. |
| [54] | A. Kamal, V. Srinivasulu, J.N.S.R.C. Murty, et al., Copper oxide nanoparticles supported on graphene oxide-catalyzed S-arylation: an efficient and ligand-free synthesis of aryl sulfides, Adv. Synth. Catal. 355 (2013) 2297-2307. |
| [55] | S. Tao, F. Gao, X. Liu, O.T. Sørensen, Preparation and gas-sensing properties of CuFe2O4 at reduced temperature, Mater. Sci. Eng. B: Solid 77 (2000) 172-176. |
| [56] | N. Taniguchi, Copper-catalyzed chalcogenation of aryl iodides via reduction of chalcogen elements by aluminum or magnesium, Tetrahedron 68 (2012) 10510-10515. |
| [57] | K.H.V. Reddy, V.R. Prakash, A.A. Kumar, G. Kranthi, Y.V.D. Nageswar, Nano copper oxide catalyzed synthesis of symmetrical diaryl sulfides under ligand free conditions, Beilstein J. Org. Chem. 7 (2011) 886-891. |
| [58] | P. Zhao, H. Yin, H. Gao, C. Xi, Cu-catalyzed synthesis of diaryl thioethers and Scycles by reaction of aryl iodides with carbon disulfide in the presence of DBU, J. Org. Chem. 78 (2013) 5001-5006. |
| [59] | F. Ke, Y. Qu, Z. Jiang, et al., An efficient copper-catalyzed carbon-sulfur bond formation protocol in water, Org. Lett. 13 (2011) 454-457. |
| [60] | B. Boduszek, J.S. Wieczorek, Synthesis of dipyridyl sulfides from pyridyl-pyridinium halides, Monatsh. Chem. 111 (1980) 1111-1116. |