b China Aviation Lithium Battery Co., Ltd., Henan 471003, China
The ion-pairing effect of ferrocene and its derivatives has been well studied in one-electron electrochemical processes [4, 5, 6], however,the ion-pairing effect in more complicated electrochemical process has not been reported yet,so the first study of multilevel ion-pairing effect in this report is of necessity and importance. Triferrocenylmethane (TriFcM),a typical three-center complex,was employed to investigate the multilevel ion-pairing effect through ‘‘thin-layer electrochemistry’’ approach. 2. Experimental 2.1. Chemicals The tetraethylammonium fluroperchlorate (TEAFP) and perchloric acid were all purchased from Alfa Aesar China Co.,Ltd.,and nitrobenzene (NB) from Alfa Aesar China Co.,Ltd. was purified consecutively with 0.1 mol/L HCl,0.1 mol/L NaOH,and deionized water. The sodium hydroxide,hydrochloric acid,and trichloromethane were all purchased from Beijing Chemical Works. Ferrocene (Fc) was from Aldrich and triferrocenylmethane (TriFcM) was provided by professor Hua-Zhong Yu at Simon Fraser University in Canada. 2.2. Apparatus and procedure Conventional electrochemical cells and instrumentation were employed for the electrochemical measurements. A cylindrical edge-plane graphite (EPG) rod sealed with heat-shrinkable tubing was employed as the working electrode,with an exposure area of 0.34 cm 2 at the edge of the graphitic planes. A platinum wire was employed as the counter electrode while Ag/AgCl/3 mol/L KCl was used for the reference electrode. The working electrode was polished with a piece of 600-grit sand paper,sonicated,and washed in deionized water,then dried with a heat gun before 1.0 mL drop of NB containing TriFcM was applied onto the surface of the working electrode. The electrochemical experiments and UV-vis spectroscopy were carried out with a CHI610D instrument and UV-5300 PC spectrophotometer,respectively. All measurements were performed at room temperature. 3. Results and discussion 3.1. Characterization of TriFcM The UV-vis spectrum of TriFcM and Fc was obtained and shown in Fig. 1(a),obviously,two distinct bands exist. The peak around 245 nm corresponding to the cyclopentadienyl ring of TriFcM is smaller than that of Fc at 260 nm,which was caused by the electron-withdrawing effect of methenyl in TriFcM [3]. The single peak around 450 nm is contributed by the characteristic d-d transition in the ferrocenyl nucleus of TriFcM,which is almost the same with Fc molecule. These indicate the virtually unchanged electronic structure for each ferrocenyl moiety of TriFcM [3].

|
Download:
|
| Fig. 1. (a) The UV-vis absorption spectrum of 0.2 mmol/L TriFcM (long dash) and 0.3 mmol/L Fc (solid) in CHCl3solvent; (b) cyclic voltammogram of 1.1 mmol/L TriFcM in thin NB film covering on the EPG electrode surface while 0.5 mol/L HClO4 was employed as supporting electrolyte in aqueous solution. The scan rate was 10 mV/s. | |
The TriFcM was investigated through thin-layer electrochemistry with 0.5 mol/L HClO4 as the supporting electrolyte in
aqueous solution. Interestingly,three pairs of reversible peaks of
TriFcM appeared,and the potential separations between two
adjacent peaks are  =0.202 V and
=0.202 V and 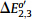 =0.134 V (shown in
Fig. 1(b)),which was thought to be caused by two factors: one is
the intramolecular electronic communication resulting from
repulsive electrostatic forces among three ferroceniums during
electrochemical process,the other is the multilevel ion-pairing
effect between ferrocenium and perchlorate in the organic phase
[4]. The whole reaction process could be demonstrated as
follows:
=0.134 V (shown in
Fig. 1(b)),which was thought to be caused by two factors: one is
the intramolecular electronic communication resulting from
repulsive electrostatic forces among three ferroceniums during
electrochemical process,the other is the multilevel ion-pairing
effect between ferrocenium and perchlorate in the organic phase
[4]. The whole reaction process could be demonstrated as
follows:

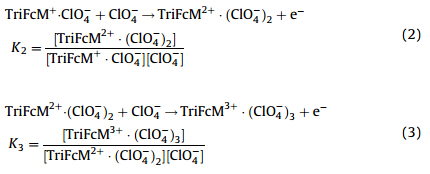

|
Download:
|
| Fig. 2. (a) CVs recorded at an EPG electrode covered with 1 mL of NB containing 1.1 mmol/L TriFcM as the concentration of HClO4 in the aqueous solution changed from 0.1 mol/L to 2.0 mol/L; (b) dependence of each voltammetric peak potential of TriFcM (corrected for the liquid junction potential,Ej) on the logarithm of concentration of ClO4 - in NB with HClO4as the supporting electrolyte in aqueous solution. | |
Based on the confirmation of ion concentration in NB phase and the liquid/liquid junction potential in our previous work [4],the multilevel ion-pairing effect of TriFcM could be evaluated through Eq.(4) [4]:
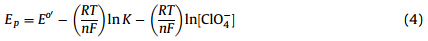
where Epis the peak potential of the symmetric oxidative wave.
To our surprise,three linear dependences of Ep(corrected for liquid junction potential Ej) for three ferrocenyl moieties of TriFcM on ln[ClO4- ] were obtained in Fig. 2(b). According to Eq. (4),three formation constants of the ion-pairs for three ferrocenyl moieties of TriFcM (K1= 1.0 × 10 7 ,K2= 1.3 × 10 7 ,K3= 1.9 × 10 7 ) were obtained through individual intercepts of Fig. 2(b). The order of K1,K2,K3 was considered to be mainly caused by multilevel electrostatic interactions between ferrocenium and perchlorate, the bigger the electrostatic interaction,the bigger the formation constant of ion-pair [4].
The intramolecular electronic communication of TriFcM during successive processes of electrochemical oxidation results in different positive charge numbers for the three ferroceniums [2, 3],which follows the order of qFc+ 1 < qFc+ 2 < qFc+ 3 : Since anion(ClO4- ) is identical in each reaction,the comparative value of electrostatic interaction between ferrocenium and perchlorate was determined by the positive charge number of ferrocenium,the more positive the charge number,the bigger the electrostatic interaction. Therefore,the multilevel formation constant of ionpair follows the above order of K1,K2,K3. 4. Conclusion TriFcM was characterized with UV-vis spectroscopy and thinlayer electrochemistry,in which the multilevel ion-pairing effect was first studied. The three one-electron electrochemical reaction process of TriFcM was determined by the intramolecular electronic communication among ferrocenyl moieties and the multilevel ionpairing effect. The multilevel ion-pairing effect of TriFcM was studied quantitatively,which conforms to the order of K1< K2< K3 resulting from different positive charge numbers of ferrocenium.
Acknowledgment The authors gratefully acknowledge the NSFC (No. 21173023), the 111 Project (No. B07012) in China and the TriFcM provided from professor Hua-Zhong Yu at Simon Fraser University in Canada.| [1] | J.K. Ouyang, L.J. Chen, L. Xu, C.H. Wang, H.B. Yang, A new family of supramolecular multiferrocenyl rhomboids: synthesis, characterization, and their electrochemical behavior, Chin. Chem. Lett. 24 (2013) 471-474. |
| [2] | J. Xu, A. Frcic, J.A.C. Clyburne, R.A. Gossage, H.Z. Yu, Thin-layer electrochemistry of 1,3-diferrocenyl-2-buten-1-one: direct correlation between driving force and liquid/liquid interfacial electron transfer rates, J. Phys. Chem. B 108 (2004) 5742-5746. |
| [3] | M.C.P. Wang, Y.C. Li, N. Merbouh, H.Z. Yu, Thin-layer electrochemistry of ferrocenylbenzene derivatives: intramolecular electronic communication, Electrochim. Acta 53 (2008) 7720-7725. |
| [4] | D.B. Xiang, G.Y. Gao, H.B. Shao, et al., Redox behavior and ion-pairing thermodynamics of ferrocene and its derivatives in the organic phase, J. Phys. Chem. C 114 (2010) 617-621. |
| [5] | E. Dionne, T. Sultana, L. Norman, V. Toader, A. Badia, Redox-induced ion pairing of anionic surfactants with ferrocene-terminated self-assembled monolayers: faradaic electrochemistry and surfactant aggregation at the monolayer/liquid interface, J. Am. Chem. Soc. 135 (2013) 17457-17468. |
| [6] | Y. Yokota, T. Yamada, M. Kawai, Ion-pair formation between ferrocene-terminated self-assembled monolayers and counteranions studied by force measurements, J. Phys. Chem. C 115 (2011) 6775-6781. |




