The charge transfer (CT) complex is formed from a weak interaction between an electron donor,having sufficient low ionization potential,and an acceptors,having sufficient high electron affinity. The transfer of an electron from a donor to an acceptor is possible in charge transfer process [1]. The formation of a CT complex is often characterized by appearance of an intense, broad electronic absorption band in UV-vis region that can be used for identification of the CT complex [2]. But,such band does not exist in the absorption spectra of both the donor and acceptor species. The CT complexes have been found applications in studying redox processes [3],optochemical sensor in organic conductors and photoconductors. Such complexes also play important roles in drug interactions and in many biological systems [4, 5, 6, 7, 8, 9, 10].
Iodine is well known for its electron-acceptor properties,which may be deduced from molecular orbital considerations [11]. It has been used in the past as a model acceptor to investigate the electron-donating properties of organic molecules. In human biology,iodine is required for the biosynthesis of the thyroid hormones,triiodothyronine and thyroxin,which regulate metabolic rate. Iodine is used in antiseptic,food supplements,dyes, catalysts,halogen lights,and photography. During the past decades,the charge transfer complex of iodine with a wide variety of molecules has been applied [12, 13].
We have been recently involved in the spectrophotometric study of interaction between ICl3,as an acceptor,with some donor ligands in different solvents at various temperatures [14].
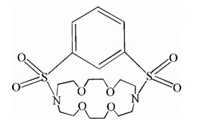
The present research aims mainly the spectrophotometric investigation of the stoichiometry and thermodynamics of complex formation of I2with benzene-1,3-disulfonylamid-kriptofix[22] in chloroform and dichloromethane solutions at different temperatures. The synthetic ligand is macrocyclic aza crown ether containing nitrogen and oxygen atoms as donor sites in its structure (BDSAK). 2. Experimental
Reagent grade iodine (I2),chloroform (CHCl3) and dichloromethane (DCM) were obtained from Merck Company (Darmstadt, Germany) with highest purity available and used without any further purification. The synthetic ligand,benzene-1,3-disulfonylamid-kriptofix[22] (BDSAK) (Scheme 1) was prepared and purified according to Ref. [15].
|
Download:
|
| Scheme 1.The structure of BDSAK. | |
The electronic absorption spectra were recorded on a PerkinElmer Lambda-45 UV-vis spectrophotometer with 1.0-cm quartz cells. For thermodynamic studies,absorbance measurements were made on a Shimadzu UV-265 spectrometer that was equipped with a temperature controlled cell holder. Both sample and blank compartment were kept at constant temperature by a thermostat which allowed the temperature to be maintained constant to ±0.1 ℃.
Fresh solution of I2 was prepared before each series of measurements by dissolving accurately weighted amounts of the component in the appropriate volume of solvent and BDSAK solution was added by a Hamilton microsyringe. 3. Results and discussion
The electronic absorption spectra of I2 (5 × 10 -4 mol L-1 ) in the presence of different concentrations of BDSAK (0.0-1.0 × 10 -2 mol L-1 ) in chloroform and dichloromethane solutions at 25 ℃ are shown in Fig. 1. Iodine solutions in chloroform and dichloromethane have an absorbance band with a λmax of 510 nm while BDSAK does not show any absorption band in the investigated region. With the addition of BDSAK as a donor to iodine solution,strong changes in color were observed and associated with the appearance of new absorption band in 368 nm region where neither donor nor acceptor have any absorption in this region. The decrease in the absorbance at 510 nm and appearance of a new band and its increase at 368 nm is due to interaction between ligand and iodine and the formation of triiodide ion (I3- ) in solution. The formation of I3- ion indicated the charge transferring complex between iodine and the ligand’s lone pair electrons. The mechanism of CT formation between the ligand and I2can be proposed as:


|
Download:
|
| Fig. 1. Absorption spectra for 1.0 × 10 -2 mol L -1 BDSAK (1),5.0 × 10 -4 mol L -1 I2 (2) in chloroform in the presence of various concentrations of BDSAK. The BDSAK/I2 are 2,0.0; 3,0.1; 4,0.2; 5,0.3; 6,0.4; 7,0.5; 8,0.6; 9,0.8; 10,1.0; 11,1.2; 12,1.4; 13, 1.6; 14,1.8; 15,2:1. | |
The above equations show the formation of CT complexes. These are contact ion pair,[((LI+ )I3- )L],and solvent separated ion pair,[((LI+ )L]+I3- ). Triiodide in the contact ion pair showed two absorption bands with the maximum absorption wavelengths of 260 and 390 nm,but these wavelengths shifted to 290 and 360 nm, respectively in the solvent separated ion pair [16, 17, 18]. In the BDSAK-I2 system the maximum absorbance wavelength for I3- was observed at 368 nm. This indicated that in the BDSAK system, a solvent separated ion pair was produced.
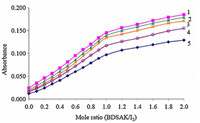
The stoichiometry of the formed complexes between I2-BDSAK was determined by applying mole ratio and continuous variations methods [19]. Plot of absorbance versus different concentrations of BDSAK mole ratio (0-2) in chloroform and dichloromethane solutions at 25 ℃ is shown in Fig. 2. The change in absorbance in the investigated region was found to increase with arising in the BDSAK:I2mole ratio and after mole ratio of 1:1,the slope of curves are very slow that can be consider as a nearly constant value.

|
Download:
|
| Fig. 2. Plots of absorbance vs. CBDSAK/CI2 mole ratio in chloroform (1) and dichloromethane (2) at 25 ℃. | |
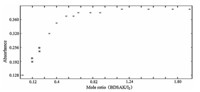
Fig. 3 shows the continuous variations plots in CHCl3and DCM and exhibits a maximum at XBDSAK value of 0.5 that emphasizes the formation of a 1:1 I2-BDSAK complex.

|
Download:
|
| Fig. 3. Continuous variation Plots of BDSAK/I2mole fraction in chloroform (1) and dichloromethane (2). | |
The formation constants of BDSAK-I2complex in both solvents were determined from change in absorbance at 368 nm,upon the mixing of BDSAK and I2in different temperatures (Figs. 4 and 5), and then were evaluated by computer fitting of the corresponding absorbance-mole ratio data by user of KINFIT program [20]. The results are listed in Table 1. A sample computer fit of the absorbance vs. BDSAK/I2data is shown in Fig. 6. As it can be seen, the fair agreement supports the existence of 1:1 complexation between the BDSAK and iodine in solutions.
|
Download:
|
| Fig. 4. Plots of absorbance vs. CBDSAK/CI2 mole ratio in chloroform at (1) 10 ℃,(2) 15 ℃,(3) 20 ℃,(4) 25 ℃,(5) 30 ℃ | |
|
Download:
|
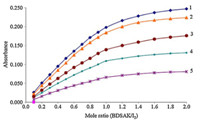
| Fig. 5. Plots of absorbance vs. CBDSAK/CI2 mole ratio in dichloromethane at (1) 10 ℃, (2) 15 ℃,(3) 20 ℃,(4) 25 ℃,(5) 30 ℃. | |
|
Download:
|
| Fig. 6. Computer fit of absorbance vs. BDSAK/I2mole ratio in dichloromethane solution at 25 ℃. (Ο) calculated point; (×) experimental point; (=) experimental and calculated points are the same within the resolution of the plots. | |
| Table 1 Stability constants of BDSAK I2 complex in different temperatures at various solvents. |
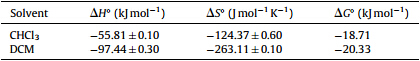
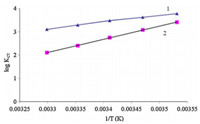
In order to have a better understanding of the thermodynamics of the complexation reactions of iodine with BDSAK,it is useful to consider the enthalpic and entheropic contributions to this reaction. Thermodynamic parameters,ΔH° and ΔS°,have been obtained from the temperature variation of stability constants using van’t Hoff’s equation. Plots of log K against 1000/T give straight lines (Fig. 7),the enthalpies and entropies of complexation were determined from the slope and intercept of plots respectively. The results are also included in Table 2. The complexation are exclusively exothermic and enthalpy driven with a negative entropic contribution. The negative enthalpies show that complex formation is spontaneous while negative entropy indicates a decrease in the degree of freedom of the components upon complexation.
|
Download:
|
| Fig. 7. Van’t Hoff Plots for (1) chloroform,(2) dichloromethane solutions. | |
| Table 2 Thermodynamic parameters of BDSAK.I2complex in different solvents |
As the complexation mechanism results specified,the CT complexes of iodine and BDSAK in two solvents are based on solvent separated ion pair. Table 1 shows that the KCT in DCM solvent are higher than chloroform. These behavior may be related to the difference in the dielectric constant of two solvents and it is not surprising that in DCM with ε = 9.1 [21],the complex formation is high stabilized than in chloroform with ε = 4.8. Also,the data given in Table 1 revealed that the KCT values vary according to the polarity of the medium. The stability of the resulting iodine complexes increase with increasing polarity of the solvent from chloroform to DCM [22, 23]. It has been suggested that the observed trend in the stability of the charge-transfer complexes could be due to the high stabilization of the exited states in which the charge is probably more separated than in the ground states. And also it is has been observed that the stability of complex decrease very significantly with increasing temperature and with increasing donor number and dielectric constant of solvent [24]. 4. Conclusion
The interaction between iodine and benzene-1,3-disulfonylamid-kriptofix[22],(BDSAK) as a synthetic ligand,in two different solvents was investigated. The stoichiometry of the complexation reaction showed that the I2:BDSAK ratio for the produced complex is 1:1. The stability constants in different temperatures were calculated and thermodynamic parameters were calculated from the dependence of the stability constants on temperature using van’t Hoff equation.
| [1] | H.B. Hassib, Y.M. Issa, Conductimetric studies of charge transfer complexes of some benzylidene aniline schiff bases with substituted p-benzoquinones, Egypt, J. Chem. 39 (1996) 329-338. |
| [2] | R.S. Mulliken, Molecular compounds and their spectra. III. The interaction of electron donors and acceptors, Phys. Chem. 56 (1952) 801-822. |
| [3] | K. Brueggermann, R.S. Czernuszewicz, J.K. Kochi, Charge-transfer structures of aromatic electron donor-acceptor complexes with titanium tetrachloride. Ground-state and excited-state spectroscopy for redox processes, Phys. Chem. 96 (1992) 4405-4414. |
| [4] | M. Goes, J.W. Verhoeven, H. Hofstraat, K. Brunner, OLED and PLED devices employing electrogenerated, intramolecular charge-transfer fluorescence, Chem. Phys. Chem. 4 (2003) 349-358. |
| [5] | A. Eyehmuller, A.L. Rogach, Chemistry and photophysics of thiol-stabilized II-VI semiconductor nanocrystals, Pure Appl. Chem. 72 (2000) 179-188. |
| [6] | R. Dabestani, K.J. Reszka, M.E. Sigman, Surface catalyzed electron transfer from polycyclic aromatic hydrocarbons (PAH) to methyl viologen dication: evidence for ground-state charge transfer complex formation on silica gel, J. Photochem. Photobiol. A 117 (1998) 223-226. |
| [7] | S.Y. AlQaradawi, E.L.M. Nour, Synthesis and spectroscopic structural studies of the adducts formed in the reaction of aminopyridines with TCNQ, J. Mol. Struct. 794 (2006) 251-254. |
| [8] | K. Wang, D.S. Guo, M.Y. Zhao, Y. Liu, A supramolecular vesicle based on the complexation of p-sulfonatocalixarene with protamine and its trypsin-triggered controllable-release properties, Chem. Eur. J. (2014), http://dx.doi.org/10.1002/ chem.201303963. |
| [9] | X. He, B. Xu, Y.Q. Liu, Y.Q. Yang, W.J. Tian, Effect of intramolecular charge transfer on the two-photon absorption behavior of multibranched triphenylamine derivations, J. Appl. Phys. 111 (2012) 053516-153516. |
| [10] | X.Y. Shen, W.Z. Yuan, Y. Liu, et al., Fumaronitrile-based fluorogen: red to near-infrared fluorescence, aggregation-induced emission, solvatochromism, and twisted intramolecular charge transfer, J. Phys. Chem. 116 (2012) 10541-10547. |
| [11] | M.J.S. Dewar, A.R. Lepley, π-Complexes. I. Charge transfer spectra of π-complexes formed by trinitrobenzene with polycyclic aromatic compounds, J. Am. Chem. Soc. 83 (1961) 4560-4563. |
| [12] | A. Afkhami, T. Madrakian, H. Tahmasebi, H. Keypour, H. Khanmohammadi, Interaction of new polyamine ligand N,N,N',N'-tetrakis(2-salicylideneaminoethyl) butane-1,4-diamine with iodine in chloroform and dichloromethane solutions, Phys. Chem. Liq. 46 (2008) 372-378. |
| [13] | T. Madrakian, M. Mohammadnejad, F. Hojati, Investigation of electron donoracceptor complex formation between morpholine and 2,4,6-trimorpholino-1,3,5-triazin with iodine in two solvents with soft-modeling approaches, J. Mol. Struct. 968 (2010) 1-5. |
| [14] | M. Torabbeigi, T. Madrakian, A. Afkhami, Kinetic study of charge transfer complexes of ICl3 with DB18C6 and DC18C6 in some nonaqueous solvents, J. Incl. Phenom. Macrocycl. Chem. 67 (2010) 127-132. |
| [15] | A. Ghaderi, M.S. Thesis, The Synthesis of Quinolines under Green Conditions, Faculty of Chemistry. Bu-Ali Sina University, Hamedan, Iran, 2010. |
| [16] | L.J. Andrew, E.S. Prochaska, A. Loewenschuss, Resonance Raman and ultraviolet absorption spectra of the triiodide ion produced by alkali iodide-iodine argon matrix reactions, Inorg. Chem. 19 (1980) 463-465. |
| [17] | M. Mizuno, J. Tanaka, I. Harada, Electronic spectra and structures of polyiodide chain complexes, J. Phys. Chem. 85 (1981) 1789-1794. |
| [18] | A. Semnani, M. Shamsipur, Spectrophotometric study of the complexation of iodine with macrocycles in chloroform solution, J. Chem. Soc. Dalton Trans. 11 (1996) 2215-2218. |
| [19] | W. Likussar, D.F. Bolts, Theory of continuous variations plots and a new method for spectrophotometric determination of extraction and formation constants, J. Anal. Chem. 43 (1971) 1265-1272. |
| [20] | V.A. Nicely, J.L. Dye, A general purpose curve fitting program for class and research use, J. Chem. Educ. 48 (1971) 443. |
| [21] | V. Gutmann, Coordination Chemistry in Nonaqueous Solvents, Springer-Verlag, Vienna, 1968. |
| [22] | A. Cipiciani, S. Satini, G. Saveth, Molecular complexes between substituted indoles and tetracyanoethylene, J. Chem. Soc. Faraday Trans. 1 75 (1979) 497-502. |
| [23] | A.M. Nour-el-Din, Charge-transfer complexes between beteroaromatic N-oxides and π-acceptors, Spectrochim. Acta A 41 (1985) 1101-1104. |
| [24] | I.M.M. Carvalho, M.H. Gehelan, The solvent effect on electronic energy transfer between excited [Ru(bpy)3]2+ donor and aromatic acceptors, J. Photochem. Photobiol. Chem. A 122 (1999) 109-113. |