Ionic liquids (ILs) are a broad class of salts that are composed of organic cations (e.g.,alkylimidazolium,alkylpyridinium,alkylmorpholinium) and inorganic or organic anions. The ILs with the unique properties of low vapor pressure,incombustibility,good thermal stability,strong dissolving ability and electrolytic conductivity could be widely applied in the fields of catalytic chemistry,organic synthesis,electrochemistry and analytical chemistry [1, 2, 3, 4]. In recent years,various methods were developed for the separation and detection of ILs [5],such as reversed-phase liquid chromatography (RPLC) [6, 7, 8, 9],ion chromatography (IC) [10], ion-pair chromatography (IPC) [11],hydrophilic interaction chromatography (HIC) [12, 13] and capillary electrophoresis (CE) [14]. Among them,RPLC has received much attention. In previous reports,the determinations of ILs principally focus on imidazolium and pyridinium cations [6, 7, 8, 9, 10, 11, 12, 13],since one of the most important advantages of these cations was strong ultraviolet (UV) absorption, which makes the detection easier. However,the determinations of cations lacking UV absorption groups (morpholinium cations) have not been reported thus far.
Detection by UV is the common detection technique in high performance liquid chromatography (HPLC) to determine the materials with a UV absorption group. To be useful,two other effective methods could be used to deal with the analytes without UV absorption in HPLC-UV. One way is derivatization. We can attach,via chemical reaction,strong UV absorption groups to the structures of the measured molecule,so as to directly detect the samples,but it is a complex method. The other method is by indirect ultraviolet (IUV) detection. The HPLC-IUV technique entails adding materials with UV absorption as background UV absorption reagents to the mobile phase to permit detection of analytes lacking UV absorption. So far,there are only a few reports focusing on HPLC-IUV [14, 15, 16],and the main objects of analysis are hydrocarbons and inorganic ions.
The aim of this work was to determine morpholinium cations without UV absorption by HPLC-IUV utilizing imidazolium ionic liquid as the background UV absorption reagent. Chromatographic separation was performed on a reversed phase C18 column using imidazolium ionic liquids-organic solvents as mobile phase. The effects of the background UV absorption reagents,imidazolium ionic liquids and organic solvents on the determination of morpholinium cations were investigated. A simple and practical HPLC-IUV method was developed for the determination of morpholinium cations. 2. Experimental
The ILs (≥99% purity) were N-methyl,ethylmorpholinium bromide ([MEMo]Br),N-methyl,propylmorpholinium bromide ([MPMo]Br), 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIm]BF4),1-propyl-3-methylimidazolium tetrafluoroborate ([PMIm]BF4),1-butyl-3-methylimidazolium tetrafluoroborate ([BMIm]BF4),1-amyl-3-methylimidazolium tetrafluoroborate ([AMIm]BF4),respectively. These ILs were purchased from Shanghai Chengjie Chemical Ltd. (Shanghai,China). 4-Aminophenol hydrochloride,5-sulfosalicylic acid,nicotinamide and phthalic acid (analytical grade) were purchased from J&K Chemical Ltd. (Beijing,China). Methanol and acetonitrile (HPLC grade) were obtained from Dikma Technologies (Shanghai,China).
Standard solutions of all morpholinium cations were prepared in 18.2 MV cm deionized distilled water from a Millipore Milli-Q water purification system (Millipore,Bedford,MA,USA). Stock standard solutions were prepared monthly. Working standard solutions were prepared daily,as needed,from the respective stock solutions. The working solutions were filtered using a 0.22 mm membrane filter before injection.
Milli-Q water was also used to prepare the mobile phases. Aqueous solutions of imidazolium ionic liquids were prepared at appropriate concentrations. The mobile phases were filtered through a 0.22 mm filter,and then degassed for 10 min using DOA-P504-BN pump (IDEX,CHI,USA).
The entire chromatographic experimentation was carried out on an Agilent 1200 HPLC system (Agilent,USA),which consisted of a quaternary pump (Model Quat pump-G1311A),a detector (Model DAD-G1315D),an autosampler injector (Model ALSG1329A),a column oven (Model TCC-G1316A) and a degasser system (Model Degasser-G1322A). The chromatographic system control and data analysis were performed using the Agilent Rev.B.04.01 workstation.
All separations were performed on a ZORBAX Eclipse XDB-C18 column (150 mm × 4.6 mm,i.d.,5 mm,Agilent,USA). The suitable mobile phase was 0.5 mmol/L [EMIm]BF4 aq. soln./methanol (80:20,v/v). The flow rate was set at 1.0 mL/min. Column temperature was 30 ℃. The injection volume was 20 mL. Indirect UV detection (210 nm) was employed. 3. Results and discussion
Since there were no UV absorption groups in the molecular structure of the morpholinium cations,so in order to detect these cations by UV detection,the background UV absorbing reagents were added to the mobile phase. As previous reported, 4-aminophenol hydrochloride [16],5-sulfosalicylic acid [17], nicotinamide [17] and phthalic acid [17] were used as common background UV absorbing reagents. In this study,0.5 mmol/L 4-aminophenol hydrochloride/methanol (80:20,v/v),0.5 mmol/L 5-sulfosalicylic acid/methanol (80:20,v/v),0.5 mmol/L nicotinamide/methanol (80:20,v/v),and 0.5 mmol/L phthalic acid/ methanol (80:20,v/v) were chosen as the mobile phases for detecting N-methyl,ethylmorpholinium cation (MEMo) and N-methyl,propylmorpholinium cation (MPMo) when the detection wavelengths were 240 nm,297 nm,268 nm and 255 nm, respectively. All the results show that the chromatographic peaks, i.e.,detector responses,of these two morpholinium cations do not appear.
Ionic liquids are ‘‘green solvents’’,and some ionic liquids,such as imidazolium ionic liquids,have UV absorption groups in their molecular structures,so we considered using imidazolium ILs as the background UV absorbing reagents. With regard to imidazolium ILs,[EMIm]BF4is commonly used,and therefore it was initially chosen as the background UV absorbing reagent since [EMIm]BF4 has strong UV absorption at 210 nm. When the determination of MEMo and MPMo was performed using [EMIm]BF4 aq. soln./methanol as mobile phase at 210 nm,we can clearly see the chromatographic peaks (responses) of the cations in the chromatogram. Therefore,in the following investigations,we use imidazolium ILs ([EMIm]BF4) as the background UV absorbing reagent.
Although the maximum absorption wavelength of [EMIm]BF4is 200 nm,the organic solvents (methanol) also have strong absorption at this wavelength. In order to avoid interferences, the detection wavelength at 210 nm was chosen since it was found that the chromatographic baseline was stable under this condition.
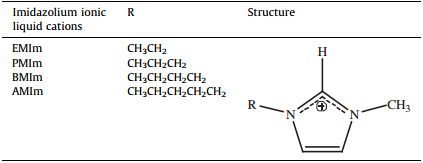
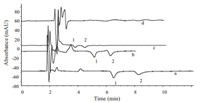
In order to investigate the influence of different imidazolium ILs with various alkyl groups on the determination of morpholinium cations MEMo and MPMo,four imidazolium ILs [EMIm]BF4, [PMIm]BF4,[BMIm]BF4and [AMIm]BF4were examined as mobile phase components,respectively. The structures of imidazolium ionic liquid cations are shown in Table 1. The mobile phases used were imidazolium ionic liquid aq. soln./methanol (80:20,v/v). As shown in Fig. 1,the retention times of MEMo and MPMo were clearly decreases as the lengths of the alkyl substituent of the imidazolium cation increases from ethyl to amyl. With growing alkyl chain lengths on the imidazole ring,the polarity of the cation is weakened and the hydrophobicity of the mobile phase is strengthened,resulting in a decreased retention time of the measured substance [18]. The separation of MEMo and MPMo was not achieved when [AMIm]BF4 is used as the mobile phase component. When [EMIm]BF4was used as mobile phase component,the chromatographic baseline noise values were less than [PMIm]BF4or [BMIm]BF4as mobile phase components. Therefore, [EMIm]BF4 was selected as the mobile phase component. The experimental results illustrated that imidazolium ILs could be,not only the background UV absorbing reagents,but also an active ingredient of the mobile phase to improve the separation of the tested substances.
| Table 1 The structures of imidazolium ionic liquid cations |
|
Download:
|
| Fig. 1. Chromatograms obtained with mobile phases containing different imidazolium ionic liquids. (a) Mobile phase,0.5 mmol/L [EMIm]BF4 aq. soln./ methanol (80:20,v/v); (b) mobile phase,0.5 mmol/L [PMIm]BF4aq. soln./methanol (80:20,v/v); (c) mobile phase,0.5 mmol/L [BMIm]BF4aq. soln./methanol (80:20,v/v); (d) mobile phase,0.5 mmol/L [AMIm]BF4 aq. soln./methanol (80:20,v/v). Chromatographic conditions: column,ZORBAX Eclipse XDB-C18 column (150 mm × 4.6 mm,i.d.,5 mm); flow rate,1.0 mL/min; column temperature,30 ℃; indirect UV detection,210 nm; inject volume,20 mL. Peaks (mg/L): 1,MEMo (31.0); 2, MPMo (32.2). | |
The effect of [EMIm]BF4concentrations on the determination of the two cations,MEMo and MPMo,was investigated using [EMIm]BF4aq. soln./methanol (80:20,v/v) as mobile phase. The concentrations of [EMIm]BF4were 0.3,0.5,0.7 and 1.0 mmol/L, respectively. When the concentrations of [EMIm]BF4are increased from 0.3 to 1.0 mmol/L,the retention times of morpholinium cations are decreased,but the chromatographic baseline noise increased. When the concentration of [EMIm]BF4is 0.3 mmol/L, peak tailing appeared. Therefore,in this study,the suitable concentration of [EMIm]BF4was 0.5 mmol/L.
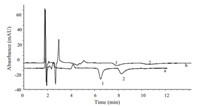
Further investigations of 0.5 mmol/L [EMIm]BF4 aq. soln./ methanol and 0.5 mmol/L [EMIm]BF4 aq. soln./acetonitrile (80:20,v/v) as mobile phases were used to determine the two morpholinium cations MEMo and MPMo,respectively. The results in Fig. 2 show that when the organic solvent is acetonitrile,the retention time of morpholinium cations is obviously longer and the phenomenon of peak tailing appeared,compared with methanol. Therefore,it is better to choose methanol as the organic component of mobile phase.
|
Download:
|
| Fig. 2. Chromatograms obtained using the mobile phases containing different organic reagents. (a) Mobile phase,0.5 mmol/L [EMIm]BF4 aq. soln./methanol (80:20,v/v); (b) mobile phase,0.5 mmol/L [EMIm]BF4aq. soln./acetonitrile (80:20, v/v). Other chromatographic conditions and peak marks are same as in Fig. 1. | |
The effects of methanol concentration on the determination of morpholinium cations were investigated by changing the volume fraction of methanol in the mobile phase from 10% to 35%,and the mobile phase was the mixed solution of 0.5 mmol/L [EMIm]BF4aq. soln.-methanol. It is shown that with increasing the volume fraction of methanol in the mobile phase,the retention time of morpholinium cations increased,as well as the peak tailing phenomenon increased. However,the baseline noise values decreased at the same time. For the simultaneous determination of MEMo and MPMo,the methanol volume fraction of 20% is appropriate.
The temperature of column was investigated with 0.5 mmol/L [EMIm]BF4aq. soln./methanol (80:20,v/v) as mobile phase,and the flow rate was 1.0 mL/min. At a column temperature of 25 ℃,the retention times of MEMo and MPMo were 6.266 and 7.961 min, respectively; when the column temperature was 30 ℃,the retention times of MEMo and MPMo were 6.374 and 8.076 min,respectively; when the column temperature was 35 ℃,the retention times of MEMo and MPMo were 6.427 and 8.137 min,respectively; when the column temperature was 40 ℃,the retention times of MEMo and MPMo were 6.468 and 8.175 min,respectively. With increasing temperature from 25 ℃ to 40 ℃,the retention times of the morpholinium cations were increased slightly,but the effects of column temperature on retention time of the cations were considered insignificant. Therefore,30 ℃ was selected as an appropriate temperature because it is close to room temperature.
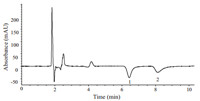
According to the above discussion,the suitable chromatographic conditions for determination of morpholinium cations are described as follows. The analyses are performed using a ZORBAX Eclipse XDB-C18 column with an IUV detection wavelength of 210 nm,0.5 mmol/L [EMIm]BF4aq. soln./methanol (80:20,v/v) as mobile phase,a flow rate of 1.0 mL/min and the column temperature controlled at 30 ℃. Under these conditions,the chromatogram of two morpholinium cations is shown in Fig. 3.
|
Download:
|
| Fig. 3. Chromatogram of a standard mixture solution of two morpholinium cations. Chromatographic conditions: column,ZORBAX Eclipse XDB-C18 column (150 mm × 4.6 mm,i.d.,5 mm); mobile phase,0.5 mmol/L [EMIm]BF4 aq. soln./ methanol (80:20,v/v); flow rate,1.0 mL/min; column temperature,30 ℃; indirect UV detection,210 nm; inject volume,20 mL. Peaks (mg/L): 1,MEMo (31.0); 2, MPMo (32.2) | |
Calibration curves,detection limits and the precision of the method were determined on a series of standard solutions of MEMo and MPMo under the established chromatographic conditions. With quantitative use of peak area,linear regression equations were obtained from the relationship between peak area (integral value) and ionic concentration (mg/L). Detection limits were calculated with a tripled signal-to-noise ratio (S/N = 3), and noise of the baseline was 0.0744 mAU. Relative standard deviations (RSD) were obtained by five repeated measurements of a standard mixture solution of MEMo and MPMo under the chromatographic conditions. The data are listed in Table 2. The results show that the reproducibility and linearity of the method can meet the requirements of quantitative analysis.
| Table 2 Linear regression equation,limits of detection (LOD) and relative standard deviation for retention time and peak area (RSDt/RSDa). |
This method was applied to the determination of morpholinium ionic liquids synthesized by the chemistry lab,namely Nmethyl,ethyl-morpholinium bromide ([MEMo]Br) and N-methyl,-propyl-morpholinium bromide ([MPMo]Br). The analysis weights of [MEMo]Br and [MPMo]Br were 0.1010 g and 0.1011 g, respectively,diluted to 100 mL as stock solutions. Then,2.5 mL [MEMo]Br,and 2.5 mL [MPMo]Br were taken from the stock solutions and diluted to 50 mL,respectively. After filtering through a 0.22 mm membrane filter,these sample solutions were used for the determination of morpholinium cations under the selected conditions. Recoveries were tested by the standard addition method. Analytical results and recoveries of morpholinium cations in the ionic liquid samples are listed in Table 3. The data in Table 3 are average values (n = 5),and the RSDs are less than 2.7%. Data from Table 3 prove the method to be accurate and reproducible, and can meet the requirements of quantitative analysis of morpholinium cations.
| Table 3 Analytical results and recoveries of morpholiniun cations found in ionic liquid samples. |
In this work,a novel approach was developed using imidazolium ionic liquids as background UV absorbents in HPLC with indirect UV detection for determination of morpholinium cations, which have no UV absorption group in their molecular structures. As mobile phase additives,the imidazolium ionic liquids can not only act as background UV absorbents,but also improve the separation of analytes. The morpholinium cations can be separated by only using imidazolium ionic liquid aq. soln.-methanol as mobile phase on a reversed-phase C18 column. This method has been successfully applied to determine morpholinium cations in ionic liquids synthesized by chemistry lab. The results were accurate,and the reproducibility was satisfactory. The method is simple,rapid,and it can meet the quantitative analysis requirements of morpholinium cations.
Acknowledgments This work was supported by the Natural Science Foundation of Heilongjiang Province (No. B201307),the Ministry of Education of Heilongjiang Province (No. 12531192) and the Program for Scientific and Technological Innovation Team Construction in Universities of Heilongjiang Province (No. 2011TD010).
| [1] | R.Q. Zhang, H. Yu, X.J. Sun, Separation and determination of pyrrolidinium ionic liquid cations by ion chromatography with direct conductivity detection, Chin. Chem. Lett. 24 (2013) 503-505. |
| [2] | T.D. Ho, C. Zhang, L.W. Hantao, J.L. Anderson, Ionic liquids in analytical chemistry: fundamentals, advances, and perspectives, Anal. Chem. 86 (2014) 262-285. |
| [3] | W. Gao, H. Yu, S. Zhou, Applications of ionic liquids in chromatographic analysis and determination of ionic liquids by chromatography, Chin. J. Chromatogr. 28 (2010) 14-22. |
| [4] | K.P. Boroujeni, M. Jafarinasab, Polystyrene-supported pyridinium chloroaluminate ionic liquid as a new heterogeneous Lewis acid catalyst for Knoevenagel condensation, J. Chem. Res. 36 (2012) 429-431. |
| [5] | M. Paszkiewicz, P. Stepnowski, How should ionic liquids be analyzed? Curr. Org. Chem. 15 (2011) 1873-1887. |
| [6] | C.M. Zou, H. Yu, M.Y. Wang, Determination of tetraethyl ammonium by ion-pair chromatography with indirect ultraviolet detection using 4-aminophenol hydrochloride as background ultraviolet absorbing reagent, Chin. Chem. Lett. 25 (2014) 201-204. |
| [7] | B. Buszewski, S. Kowalska, P. Stepnowski, Influence of stationary phase properties on the separation of ionic liquid cations by RP-HPLC, J. Sep. Sci. 29 (2006) 1116-1125. |
| [8] | M.J. Ruiz-Angel, A. Berthod, Reversed phase liquid chromatography of alkylimidazolium ionic liquids, J. Chromatogr. A 1113 (2006) 101-108. |
| [9] | M.J. Ruiz-Angel, A. Berthod, Reversed-phase liquid chromatography analysis of alkyl-imidazolium ionic liquids II. Effects of different added salts and stationary phase influence, J. Chromatogr. A 1189 (2008) 476-482. |
| [10] | W. Gao, H. Yu, Determination of homologue imidazolium ionic liquid cations by ion chromatography using a carboxyl acid cation-exchange column with direct conductivity detection, Anal. Lett. 44 (2011) 922-931. |
| [11] | X. Huang, H. Yu, Y.J. Dong, Rapid and simultaneous determination of imidazolium and pyridinium ionic liquid cations by ion-pair chromatography using a monolithic column, Chin. Chem. Lett. 23 (2012) 843-846. |
| [12] | G.L. Rouzoa, C. Lamourouxb, C. Bresson, et al., Hydrophilic interaction liquid chromatography for separation and quantification of selected room-temperature ionic liquids, J. Chromatogr. A 1164 (2007) 139-144. |
| [13] | C. Lamouroux, G. Foglia, G. Le Rouzo, How to separate ionic liquids: use of hydrophilic interaction liquid chromatography and mixed mode phases, J. Chromatogr. A 1218 (2011) 3022-3028. |
| [14] | M. Shi, Q.Y. Gao, J.M. Feng, Y.C. Lu, Analysis of inorganic cations in honey by capillary zone electrophoresis with indirect UV detection, J. Chromatogr. Sci. 50 (2012) 547-552. |
| [15] | G. Schill, E. Arvidsson, Application of indirect detection methods in biomedical analysis, J. Chromatogr. 492 (1989) 299-318. |
| [16] | X.H. Fan, Spectrophotometric determination of iron in agricultural samples with pyrogallol red in the presence of CPB, Technol. Dev. Chem. Ind. 31 (2002) 34-35. |
| [17] | L.N. Li, Z.K. Duan, H.Y. Zeng, Y.P. He, Q.Y. Chen, Determination of hexane in the acid cluster of caprolactam by high performance liquid chromatography with indirect photometric detection, Chin. J. Chromatogr. 29 (2011) 87-90. |
| [18] | Y. Polyakova, K.H. Row, Retention behaviour of N-CBZ-D-phenylalanine and Dtryptophan: effect of ionic liquid as mobile-phase modifier, Acta Chromatogr. 17 (2006) 210-221. |