b Engineering Research Center of Biomass Materials, Ministry of Education, School of Material Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, China;
c Research Center of Laser Fusion, China Academy of Engineering Physics, Mianyang 621900, China
Perylene bisimides (PBIs) have attracted great interest due to their intense luminescence,high chemical stability and thermal stability [1, 2, 3, 4]. Consequently,more and more interesting investigations have been focused on the modification of perylene bisimide structures through high-yield synthetic routes to improve the chemical and physical properties [5, 6, 7, 8, 9, 10, 11]. Although considerable research has been conducted on the systems containing PBIs [12, 13, 14, 15, 16, 17, 18],studies on the benzocyclobutene-perylene system have rarely been reported.
Benzocyclobutene (BCB) is a reactive intermediate which can react either with itself,or the appropriate dienophiles to obtain the corresponding Diels-Alder products at appropriate temperatures [19, 20]. When heated to approximately 200 ℃,the cyclobutene ring of BCB opens and forms a highly reactive o-quinodimethane intermediate. If a dienophile is present,the o-quinodimethane intermediate can react to form stable Diels-Alder adducts. On the other hand,the intermediate can also form an unstable spirodimer which rearranges to a polymeric material. The BCB group can also be incorporated into polymers through other functional groups to provide reactive sites [21].
The preparation of most functional materials is based on the simple mixing of the required functional components [22, 23]. In this letter,we synthesize the molecule which contains four benzocyclobutene units tethered to a perylene bisimide dye and the result demonstrate that the benzocyclobutene units can undergo Diels-Alder polymerization and cross-linking reactions by thermal isomerization to form a polymeric network. This approach seems to be quite promising as it allows incorporation of two classes of compounds that possess the thermal polymerization properties of benzocyclobutene [24, 25] and optical properties of perylene bisimide [26, 27] within a single,easily accessible material. 2. Experimental
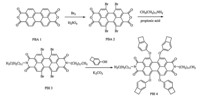
The 1,6,7,12-tetrabromoperylene-3,4:9,10-tetracarboxylic acid bisanhydride (PBA 2) and N,N 0 -didodecyl-1,6,7,12-tetrabromoperylene-3,4,9,10-tetracarboxylic acid bisimide (PBI 3) were synthesized according to the literature [7].
Preparation of N,N' -didodecyl-1,6,7,12-tetra(4-benzocyclobutenyloxy)perylene-3,4,9,10-tetracarboxylic acid bisimide (PBI 4): PBI 3 (0.32 g,0.3 mmol),4-benzocyclobutenol (0.36 g,3 mmol) and potassium carbonate (0.4 g,3 mmol) were stirred in 40 mL N-methyl-2-pyrrolidone under N2 at 90 ℃ for 72 h. After being cooled to room temperature,10% hydrochloric acid (100 mL) was added,and the precipitate was collected,washed with water and methanol. The crude product was purified by silica gel column chromatography (petroleum ether:dichloromethane = 2:1) to yield PBI 4 as a red-brown solid (0.23 g,65%). 1H NMR (300 MHz,CDCl3,25 ℃,TMS): δ 8.12 (s,4H),6.95 (d,4H, J = 7.6 Hz),6.79 (d,4H,J = 7.4 Hz),6.68 (s,4H),4.09 (s,4H),3.11 (d,16H,J = 9.9 Hz),1.65 (s,4H),1.24 (m,36H),0.86 (s,6H); 13 C NMR (75 MHz,CDCl3,25 ℃,TMS): δ 163.46,156.57,154.49, 146.89,141.82,132.77,124.00,122.41,120.04,119.55,119.35, 119.11,115.51,40.62,31.89,29.70,29.59,29.51,29.31,29.05, 28.95,28.09,27.11,22.66,14.09; MS (FAB + ) (m/z): calcd. for C80H82N2O8: 1198.6071; found: 1198.6101. 3. Results and discussion
The drawback of perylene bisimides is the inherent low solubility,so the utilization is hindered. Driven by the demands in applications,the structure of perylene bisimides has been modified by introducing substituent groups either to the imide nitrogen atoms,or at the bay positions (i.e.,1,6,7,12 position) [8, 28, 29, 30]. A dodecyl group is appended at the terminal imide, primarily to enhance solubility. Benzocyclobutenyloxy groups are introduced in the bay region,and,as a result,not only the solubility and optical properties are influenced,but also allows the new product to possess thermal polymerization properties. These substitutions yield the desired molecule N,N 0 -didodecyl-1,6,7,12-tetra(4-benzocyclobutenyloxy)perylene-3,4:9,10-tetracarboxylic acid bisimide (PBI 4) (Scheme 1). The solubility of PBI 4 was determined by the dissolution of 10 mg of solid PBI in 1 mL of organic solvent at room temperature. PBI 4 is highly soluble in conventional solvents,such as toluene,chloroform (CHCl3), dichloromethane (CH2Cl2) and tetrahydrofuran (THF),and partially soluble in some organic solvents,such as N-methyl-2-pyrrolidone (NMP),acetone and dimethylformamide (DMF). Due to the good solubility of PBI 4,purification and full characterization are easy to perform.
|
Download:
|
| Scheme 1.The route for preparation of benzocyclobutene-functionalized perylene bisimide. | |
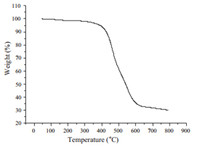
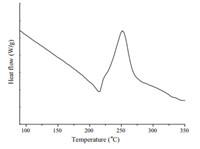
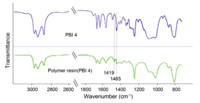
It is known that benzocyclobutene can react either with itself, or the appropriate dienophiles to produce the corresponding products by opening the cyclobutene ring under appropriate temperatures,so the thermal properties of PBI 4 were investigated by thermogravimetric analysis and differential scanning calorimetry. Based on Fig. 1,PBI 4 possesses excellent thermal stability with the initial decomposition temperature of about 344 ℃. Meanwhile,at over 800 ℃,the char yield is still 30%. The DSC spectrum of PBI 4 is shown in Fig. 2. The initial ring opening temperature occurs at about 228 ℃,and the maximum ring opening temperature of cyclobutene peaks at about 250 ℃. PBI 4 can also react with itself to form a polymeric network under appropriate temperature. Polymer resin (PBI 4) was obtained in a polymerization tube,which was sealed under N2 and heated at 250 ℃ for 6 h after three freeze-pump-thaw cycles. The IR spectra are depicted in Fig. 3. The peak at 1465 cm-1 is assigned to the vibrations of -CH2- of the alicyclic moiety. When heated to around 250 ℃,the cyclobutene ring of PBI 4 opens and forms a stable polymer resin (the peak at 1492 cm-1 ) with itself. At the same time,the peak at 1465 cm-1 does not disappear completely in the IR spectra of the polymer resin,indicating that there are still traces,or small amounts of four member rings in the polymer.
|
Download:
|
| Fig. 1. TGA curve of PBI 4. | |
|
Download:
|
| Fig. 2. DSC analysis of PBI 4. | |
|
Download:
|
| Fig. 3. IR spectra of PBI 4 (blue) and polymer resin (green) from PBI 4. | |
PBI 4 can also react with the appropriate dienophiles to form a polymeric network under appropriate temperature. A mixture of PBI 4 with methyl vinyl silicone rubber was heated at 220 ℃ for about 72 h in 1,3,5-trimethylbenzene to form the oligomer. Alternatively,the oligomer film was prepared through the spincoating technique. Then,the film was placed in polymerization tube and heated at 250 ℃ for 6 h to form the polymer film. The polymer film possesses high surface flatness. RMS is 1.245 nm. Meanwhile,the thickness of the thin-film is 62 nm by measuring of step profiler. Only blending PBI 4 with methyl vinyl silicone rubber and spin-coating them on the glass,did phase separation occur. However,the same phenomenon was not found during the spincoating process of the oligomer,and the oligomer could dissolve in the 1,3,5-trimethylbenzene. Due to crosslinking reaction,the polymer film was difficult to dissolve in any solvent. These phenomena indicated that the oligomer with a lower degree of crosslinking had been formed.
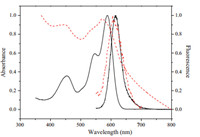
The optical properties of PBI 4 and the polymer film obtained from PBI 4 with methyl vinyl silicone rubber are investigated. Fig. 4 shows the UV/vis absorption and fluorescence spectroscopy of PBI 4 and polymer film. There are two PBI core absorption bands with maximum absorption at 589 and 454 nm in the spectrum of PBI 4, respectively,along with one shoulder absorption at 548 nm. Comparatively,the spectrum of the polymer film exhibits the distribution with maximum absorption at 574 and 448 nm,as well as the shoulder absorption at 530 nm. Although the characteristic absorption behavior of perylene bisimide compounds could be found in PBI 4 and the polymer film,the absorption bands of the polymer film undergo a blue shift compared with PBI 4. In the case of fluorescence spectroscopy,the maximum emissions of PBI 4 and the polymer film are found at about 614 and 605 nm,respectively. They are both located in the red region. The polymer film exhibits a blue shift of the emission band compared with PBI 4. These results could be attributed to the stacking of PBI moiety,which affect strongly their optical properties.
|
Download:
|
| Fig. 4. UV/vis absorption of PBI 4 (dark,solid) and polymer film (red,dash) from PBI 4 with methyl vinyl silicone rubber and fluorescence emission spectra of PBI 4 (dark,solid) and polymer film (red,dash). | |
We have presented herein a readily available benzocyclobutene-functionalized perylene bisimide,which exhibits promising thermal polymerization and optical properties. The results of thermopolymerization behavior of PBI 4 show that it can react either with itself,or methyl vinyl silicone rubber to form the corresponding products at 250 ℃. The benzocyclobutenefunctionalized perylene bisimide is a promising material,and further investigations and related benzocyclobutene-functionalized perylene bisimide materials are still underway.
Acknowledgments This work was supported by the Science and Technology Development Foundation of China Academy of Engineering Physics (Nos. 2012A0302015,2012B0302050 and 2013B0302051) and Doctoral Fund of Southwest University of Science and Technology (No. 13zx7133).| [1] | A. Hermann, K. Müllen, From industrial colorants to single photon sources and biolabels: the fascination and function of rylene dyes, Chem. Lett. 35 (2006) 978-985. |
| [2] | F. Würthner, Perylene bisimide dyes as versatile building blocks for functional supramolecular architecures, Chem. Commun. 14 (2004) 1564-1579. |
| [3] | C. Backes, F. Hauke, A. Hirsch, The potential of perylene bisimide derivatives for the solubilization of carbon nanotubes and grapheme, Adv. Mater. 23 (2011) 2588-2601. |
| [4] | R.K. Dubey, A. Efimov, H. Lemmetyinen, 1,7-and 1,6-regioisomers of diphenoxy and dipyrrolidinyl substituted perylene diimides: synthesis, separation, characterization, and comparison of electrochemical and optical properties, Chem. Mater. 23 (2011) 778-788. |
| [5] | K. Mahata, P.D. Frischmann, F. Würthner, Giant electroactive M4L6 tetrahedral host self-assembled with Fe(Ⅱ) vertices and perylene bisimide dye edges, J. Am. Chem. Soc. 135 (2013) 15656-15661. |
| [6] | Z. Chen, A. Lohr, C.R. Saha-Möller, F. Würthner, Self-assembled p-stacks of functional dyes in solution: structural and thermodynamic features, Chem. Soc. Rev. 38 (2009) 564-584. |
| [7] | L. Zhang, Y. Xu, F. Zhu, J. Sun, Synthesis and characterization of thermally-stable and soluble perylene bisimide, Asian J. Chem. 22 (2010) 7135-7144. |
| [8] | Y. Shi, H. Qian, Y. Li, W. Yue, Z. Wang, Copper-mediated domino process for the synthesis of tetraiodinated di(perylene bisimide), Org. Lett. 10 (2008) 2337-2340. |
| [9] | C. Zhao, Y. Zhang, R. Li, Y. Li, J. Jiang, Di(alkoxy)-and di(alkylthio)-substituted perylene-3,4;9,10-tetracarboxy diimides with tunable electrochemical and photophysical properties, J. Org. Chem. 72 (2007) 2402-2410. |
| [10] | P. Osswald, F. Würthner, Effects of bay substituents on the racemization barriers of perylene bisimides: resolution of atropo-enantiomers, J. Am. Chem. Soc. 129 (2007) 14319-14326. |
| [11] | Y. Avlasevich, C. Li, K. Müllen, Synthesis and applications of core-enlarged perylene dyes, J. Mater. Chem. 20 (2010) 3814-3826. |
| [12] | L. Feng, Z. Chen, Synthesis and photoluminescent properties of polymer containing perylene and fluorene units, Polymers 46 (2005) 3952-3956. |
| [13] | Y. Liu, C. Yang, Y. Li, et al., Synthesis and photovoltaic characteristics of novel copolymers containing poly(phenylenevinylene) and triphenylamine moieties connected at 1,7 bay positions of perylene bisimide, Macromolecules 38 (2005) 716-721. |
| [14] | G. Boobalan, P.M. Imran, S. Nagarajan, Synthesis of highly fluorescent and water soluble perylene bisimide, Chin. Chem. Lett. 23 (2012) 149-153. |
| [15] | X. He, H. Liu, Y. Li, et al., A new copolymer containing perylene bisimide and porphyrin moieties: synthesis and characterization, Macromol. Chem. Phys. 206 (2005) 2199-2205. |
| [16] | Q. Zhang, A. Cirpan, T.P. Russell, T. Emrick, Donor-acceptor poly(thiophene-blockperylene diimide) copolymers: synthesis and solar cell fabrication, Macromolecules 42 (2009) 1079-1082. |
| [17] | R. Ponnapati, M.J. Felipe, R. Advincula, Electropolymerizable terthiophene-terminated poly(arylether)dendrimers with naphthalene and perylene cores, Macromolecules 44 (2011) 7530-7537. |
| [18] | R. Weegen, P.A. Korevaar, P. Voudouris, et al., Small sized perylene-bisimide assemblies controlled by both cooperative and anti-cooperative assembly processes, Chem. Commun. 49 (2013) 5532-5534. |
| [19] | T. Endo, T. Koizumi, T. Takata, K. chino, Synthesis of poly(4-vinylbenzocyclobutene) and its reaction with dienophiles, J. Polym. Sci. Part A: Polym. Chem. 33 (1995) 707-715. |
| [20] | Y. Kim, J. Pyun, J.M.J. Fréchet, C.J. Hawker, C.W. Frank, The dramatic effect of architecture on the self-assembly of block copolymers at interfaces, Langmuir 21 (2005) 10444-10458. |
| [21] | J.M. Warakomski, W.C. Pike, R.A. Devries, Benzocyclobutenes as styrene monomer scavengers and molecular weight “stabilizers” in atactic and syndiotactic polystyrenes, J. Appl. Polym. Sci. 78 (2000) 2008-2015. |
| [22] | L. Schmidt-Mende, A. Fechtenkötter, K. Müllen, et al., Self-organized discotic liquid crystals for high-efficiency organic photovoltaics, Science 293 (2001) 1119-1122. |
| [23] | C. You, C.R. Saha-Möller, F. Würthner, Synthesis and electropolymerization of novel oligothiophene-functionalized perylene bisimides, Chem. Commun. 18 (2004) 2030-2031. |
| [24] | C.R. Hickenboth, J.S. Moore, S.R. White, et al., Biasing reaction pathways with mechanical force, Nature 446 (2007) 423-427. |
| [25] | E. Harth, B.V. Horn, V.Y. Lee, et al., A facile approach to architecturally defined nanoparticles via intramolecular chain collapse, J. Am. Chem. Soc. 124 (2002) 8653-8660. |
| [26] | X. Zhang, S. Rehm, M.M. Safont-Sempere, F. Würthner, Vesicular perylene dye nanocapsules as supramolecular fluorescent pH sensor systems, Nat. Chem. 1 (2009) 623-629. |
| [27] | W. Qiu, S. Chen, X. Sun, Y. Liu, D. Zhu, Suzuki coupling reaction of 1,6,7,12-tetrabromoperylene bisimide, Org. Lett. 8 (2006) 867-870. |
| [28] | A. Wicklein, A. Lang, M. Muth, M. Thelakkat, Swallow-tail substituted liquid crystalline perylene bisimides: synthesis and thermotropic properties, J. Am. Chem. Soc. 131 (2009) 14442-14453. |
| [29] | H. Wang, H. Su, H. Qian, Z. Wang, A. Xia, Structure-dependent all-optical switching in graphene-nanoribbon-like molecules: fully conjugated tri(perylene bisimides), J. Phys. Chem. 114 (2010) 9130-9135. |
| [30] | M.C.R. Delgado, E.G. Kim, D.A.S. Filho, J.L. Bredas, Tuning the charge-transport parameters of perylene diimide single crystals via end and/or core functionalization: a density functional theory investigation, J. Am. Chem. Soc. 132 (2010) 3375-3387. |




