b School of Pharmaceutical Sciences, Nanjing Tech University, Nanjing 211816, China
Multi-component reactions (MCRs) represent an attractive synthetic strategy to effectively build small-molecule libraries [1]. The possibility of increasing yields,saving both reagents and solvents,and the simplification of the purification process are among the benefits in using MCRs in organic synthesis [2]. MCRs have attracted much interest of synthetic chemists and been the focus of many synthetic efforts,especially in drug discovery and library synthesis. We have previously reported the application of MCRs to the synthesis of several types of heterocycles such as pyridine dicarbonitriles,thiazoles,and oxazoles [3].
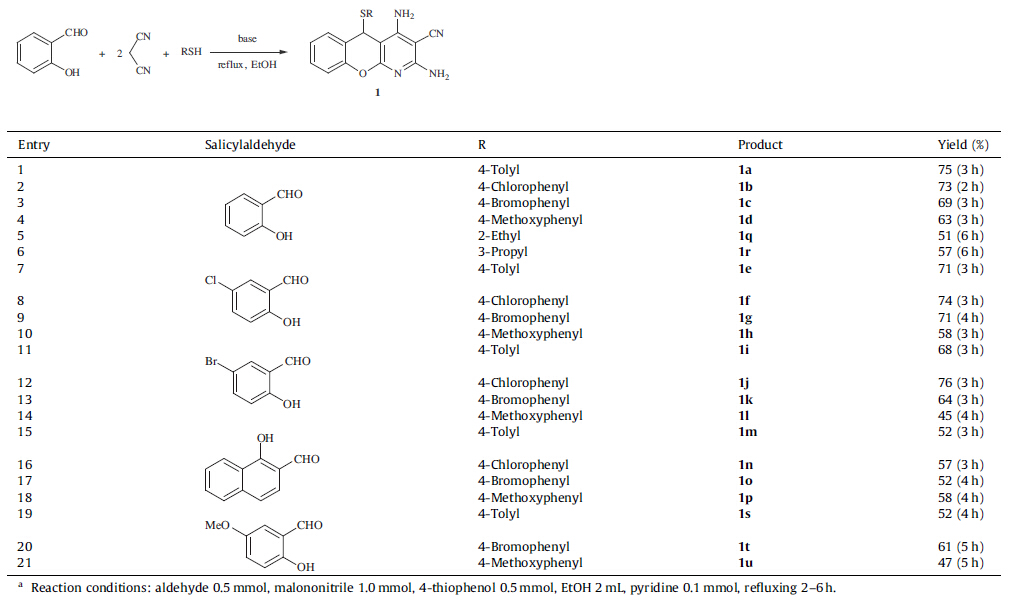
Chromenopyridine derivatives 1,which can be sucessfully synthesized via a known MCR by refluxing salicylaldehyde,thiol and malononitrile (Scheme 1) in ethanol [4],are of significant medicinal relevance. Structures bearing this motif display a diverse range of biological properties including glucocorticoid receptor (GR) agonists [5a],anti-tumor [5b],anti-bacterial [5b],anti-proliferative [5c],anti-myopic [5d],hypotensive [4a],anti-histaminic [5f],anti- rheumatic [5g],and anti-asthmatic activities [5h].
|
Download:
|
| Scheme 1.Synthesis of chromenopyridines and chromenes. | |
Chromene derivatives 2,which are widely present in plants including edible vegetables and fruits [6],represent a significant class of compounds with broad and remarkable biological active, such as antimicrobial and antifungal [7],antioxidant [8],antileishmanial [9],antitumor [10],hypotensive [11],antiproliferation [12],local anesthetic [13],antiallergenic [14],central nervous system (CNS) activities [15],as well as for the treatment of Alzheimer's disease [16] and schizophrenia disorders [17].
Several methods have been reported for the synthesis of new chromenopyridines and chromenes [4, 18]. Recently,in our study of the MCR to produce chromenopyridine,an interesting temperature- sensitivity phenomenon was observed. Chromenopyridine 1 was generated at reflux condition,while chromene 2 was obtained at room temperature condition (Scheme 1). Therefore,we conducted library synthesis and mechanistic study of the MCR. 2. Experimental
Melting point (mp) was measured on a microscopic melting point apparatus. The IR spectra were recorded on a Bruker Tensor 27 FT-IR spectrometer with a KBr disk. 1H NMR and 13C NMR spectra were taken on a Bruker AV 300 or AV 500 MHz and 75 MHz or 125 MHz spectrometer in DMSO-d6 or CDCl3,chemical shift are given in part per million (ppm) relative to TMS as an internal standard. Mass spectra and high resolution mass spectra were performed on Agilent Q TOF 6520 mass spectrometer with electron spray ionization (ESI) as the ionization mode. Optical rotation was recorded using a sodium lamp with a Rudolph Autopol I automatic polarimeter with 1 dm tube.
General procedure for the synthesis of chromenopyridines 1: Salicylaldehyde (0.5 mmol),malononitrile (1.0 mmol),thiol (0.5 mmol) were dissolved in EtOH (2 mL) and pyridine (0.1 mmol) was added and stirred at reflux temperature for 2-6 h. The reaction mixture was cooled to room temperature,filtered,washed with water and redissolved in DMF (2 mL),the undissolved marerial in DMF was filtered off,and water (4 mL) was added to the filtrate. The resulting precipitate was filtered and dried in vacuum to afford chromenopyridine (1a-u) as an off-white powder.
General procedure for the synthesis of chromenes 2: Salicylaldehyde (0.5 mmol),malononitrile (1.0 mmol),thiophenol (0.5 mmol) were dissolved in EtOH (2 mL) and the piperidine (0.1 mmol) was added and stirred at room temperature for 1-3 h. The white solid which precipitated was filtered,washed with ethanol and dried in vacuum to afford chromenes (2a-q) as an offwhite powder. The structures of the synthesized compounds were characterized by their IR,1H NMR,13C NMR and HRMS spectra. The physical and spectral data of the products are given as Supporting information. 3. Results and discussion
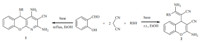
Detailed results determined by the screening of reaction condition are summarized in Supporting information. Libraries of chromenopyridines (Table 1) and chromenes (Table 2) were synthesized to prove the general applicability of the optimal reaction conditions. It was observed that all reactions employing aromatic thiols generated the desired products in both libraries. On the other hand,aliphatic thiols showed a lower reactivity. Yields of 51% and 59% were observed with ethanethiol and propanethiol as nucleophiles in Table 1 (entries 5-6),however no reaction was observed with aliphatic thiols in Table 2. The lack of reactivity of aliphatic thiols in the MCR is potentially due to the pKa of the proton in aliphatic thiols is higher than in aromatic thiols,making deprotonation of aromatic thiols easier at the same temperature. A similar phenomenon was observed for different aromatic thiols. The thiols containing an electron-donating group (such as pmethoxy) have higher pKas also lead to lower yields (entries 4,10, 14 in Tables 1 and 2). Salicylaldehyde containing an electrondonating group and 2-hydroxy-1-naphthaldehyde afforded lower yields (entries 15-21 in Table 1 and entries 13-17 in Table 2). Clearly,this is due to 6-substitution in the salicylaldehyde building block leads to more steric hindrance than 5-substitution,which was also observed in our previous research into the synthesis of pyridine-3,5-dicarbonitriles [3a, b]. The structures of 1q and 2a (Fig. 1) were confirmed by X-ray crystallography.
| Table 1 Synthesis of chromenopyridines 1.a |
| Table 2 Synthesis of chromenes 2.a |
|
Download:
|
| Fig. 1. Crystal X-ray crystallographys of compound 1q and 2a. | |
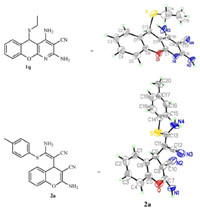
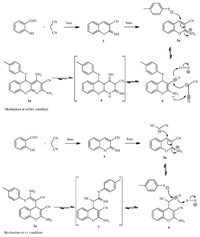
Scheme 2 elucidates the stepwise mechanism of the MCR at both reflux condition and r.t. conditions in which a chromene intermediate 3 was generated from salicylaldehyde and malononitrile in the first stage [19]. Interestingly,temperaturesensitivity selectivity was observed in the second stage of the MCR. Intermediates 4 and 6,which are 1,4-addition products of 3 with thiol and malononitrile at reflux and r.t. temperature reaction conditions respectively,were isolated. After manolonitrile was added to 4 and 4-methylthiophenol added to 6,products 1a and 2a were obtained. Hereby,the reaction of 4 and malononitrile was a synergistic ring-formed pathway at refluxing condition,while it was a nucleophilic addition between 6 and thiol at room temperature condition,resulting in different products at different temperatures. These results suggest that the reaction temperature is instrumental in controlling the precise order in which building blocks are added to the scaffold.
|
Download:
|
| Scheme 2.Mechanistic study of the MCR. | |
To further confirm the effect of temperature on formation of product,two sets of reactions were set up to explore the distribution of chromenopyridine and chromene products up to temperature in 1 h (Fig. 2). Both pyridine and piperidine were employed. In general, formation of chromenopyridine was enhanced with increasing temperature,while formation of chromene was inhibited at higher temperature,again proving that the MCR is a temperaturesensitivity reaction. Interestingly,the optimized catalyst not only improves the yield of the desired product,but also impedes formation of the other structure. Pyridine stops the formation of chromene product from 70 ?,while chromenopyridine was not formed at temperatures below 55 ? with piperidine as catalyst. In addition,the two products are not interconverted,when we subject 2a to reflux condition and find it cannot convert to 1a.

|
Download:
|
| Fig. 2. Distribution of compound 1a and 2a up to temperature in different catalysts. (a) Pyridine as catalyst; (b) piperidine as catalyst. | |
DFT calculations using Gaussian 09 [20] were performed using the B3LYP functional [21] to elucidate the experimental findings using 6-311++G (d,p) as the basis set [22]. The polarizable continuum model [23] was used to model ethanol. The calculations focused on the bifurcation point in the MCR,given that both at reflux and at r.t. intermediate 3 is formed first. Starting from intermediate 3 our calculations show that initially protonating the imide group is crucial in forming a stable intermediate upon addition of either the malononitrile or thiolate anions.
Inspection of the electrostatic potential of the protonated intermediate shows that the positive charge is located on the imine group,therefore,forming either 4 or 6 (Fig. 3) from this requires shielding of this site by the solvent and,thereby rationalizing the need for a polar,hydrogen bonding solvent for the maximum yield. The consequence for our theoretical study is that explicit solvent molecules are needed,which gives complications which are outside the scope of this paper.

|
Download:
|
| Fig. 3. Intermediates in the MCR. Panel (a) intermediate 4; panel (b) intermediate 6. | |
Analogous to the formation of 4 and 6,the subsequent formation of further intermediates in route to 1a or 2a can be rationalized by successive protonation of intermediates followed by reaction with either the malononitrile or thiolate anions, rationalizing the need for a base,which is neither too strong nor too weak. These steps were not studied in detail. Instead,the final products 1a and 2a were optimized. Our calculations show that the Gibbs energy of 1a is 126.2 kJ/mol below that of 2a,again confirming that it is the thermodynamically controlled product. 4. Conclusion
In summary,temperature-sensitivity MCRs for preparing chro- menopyridine at reflux and chromene at r.t. reaction condition were established. Libraries based on optimized reaction conditions for each structure were synthesized. Mechanistic studies proved that constructing order of building blocks was changed with different reaction temperature,which eventually lead to the formation of different products. Further investigation about biological activity of products is now underway and will be reported in due course. Acknowledgments
This work was financially supported by the National High Technology Research and Development Program of China (863 Program,No. 2012AA02A701),the National High Technology Research and Development Program of China (863 Program,No. 2013AA031901) and Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1066). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.008.
| [1] | (a) R.V.B. Orrum, M. Greep, Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds, Synthesis (2003) 1471-1499;(b) A. Döling, I. Ugi, Multicomponent reactions with isocyanides, Angew. Chem. Int. Ed. 39 (2000) 3168-3210. |
| [2] | (a) V. Nair, A.U. Vinod, C. Rajesh, A novel synthesis of 2-aminopyrroles using a three-component reaction, J. Org. Chem. 66 (2001) 4427-4429;(b) B. List, C. Castello, A novel proline-catalyzed three-component reaction of ketones, aldehydes, and Meldrum's acid, Synlett (2001) 1687-1689. |
| [3] | (a) K. Guo, M.J. Thompson, T.R.K. Reddy, R. Mutter, B. Chen, Mechanistic studies leading to a new procedure for rapid, microwave assisted generation of pyridine-3,5-dicarbonitrile libraries, Tetrahedron 63 (2007) 5300-5311;(b) K. Guo, M.J. Thompson, B. Chen, Exploring catalyst and solvent effects in the multicomponent synthesis of pyridine-3,5-dicarbonitriles, J. Org. Chem. 74 (2009) 6999-7006;(c) M.J. Thompson, B. Chen, Ugi reactions with ammonia offer rapid access to a wide range of 5-aminothiazole and oxazole derivatives, J. Org. Chem. 74 (2009) 7084-7093;(d) M.J. Thompson, J.M. Hurst, B. Chen, Regioselective, solvent-free synthesis of 3-aminoimidazo[1,2-a]pyrimidines under microwave irradiation promoted by zeolite HY, Synlett (2008) 3183-3187. |
| [4] | (a) N.M. Evdokimov, A.S. Kireev, A.A. Yakovenko, et al., One-step synthesis of heterocyclic privileged medicinal scaffolds by a multicomponent reaction of malononitrile with aldehydes and thiols, J. Org. Chem. 72 (2007) 3443-3453;(b) M. Costa, F. Areias, L. Abrunhosa, A. Venãncio, F. Proencüa, The condensation of salicylaldehydes and malononitrile revisited: synthesis of new dimeric chromene derivatives, J. Org. Chem. 73 (2008) 1954-1962;(c) S. Mishra, R. Ghosh, K2CO3-mediated, one-pot, multicomponent synthesis of medicinally potent pyridine and chromeno[2,3-b]pyridine scaffolds, Synth. Commun. 42 (2012) 2229-2244. |
| [5] | (a) D.S. Weinstein, H. Gong, A.M. Doweyko, et al., Azaxanthene based selective glucocorticoid receptor modulators: design, synthesis, and pharmacological evalu-ation of (S)-4-(5-(1-((1,3,4-thiadiazol-2-yl)amino)-2-methyl-1-oxopropan-2-yl)-5H-chromeno[2,3-b]pyridin-2-yl)-2-fluoro-N,N-dimethylbenzamide (BMS-776532) and its methylene homologue (BMS-791826), J. Med. Chem. 54 (2011) 7318-7333;(b) G. Kolokythas, N. Pouli, P. Marakos, H. Pratsinis, D. Kletsas, Design, synthesis and antiproliferative activity of some new azapyranoxanthenone aminoderivatives, Eur. J. Med. Chem. 41 (2006) 71-79;(c) M.A. Azuine, H. Tokuda, J. Takayasu, et al., Cancer chemopreventive effect of phenothiazines and related tri-heterocyclic analogues in the 12-O-tetradeca-noylphorbol-13-acetate promoted Epstein-Barr virus early antigen activation and the mouse skin two-stage carcinogenesis models, J. Pharmacol. Res. 49 (2004) 161-169;(d) S.K. Srivastava, R.P. Tripathi, R. Ramachandran, NAD+-dependent DNA ligase (Rv3014c) from Mycobacterium tuberculosis: crystal structure of the adenylation domain and identification of novel inhibitors, J. Biol. Chem. 280 (2005) 30273-30281;(e) H. Brötz-Oesterhelt, I. Knezevic, S. Bartel, et al., Specific and potent inhibition of NAD+-dependent DNA ligase by pyridochromanones, J. Biol. Chem. 278 (2003) 39435-39442;(f) D.R. Anderson, S. Hegde, E. Reinhard, et al., Aminocyanopyridine inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2), Bioorg. Med. Chem. Lett. 15 (2005) 1587-1590;(g) J.A. Bristol, E.H. Gold, I. Gross, R.G. Lovey, J. Long, Gastric antisecretory agents. 2. Antisecretory activity of 9-[(aminoalkyl)thio]-9H-xanthenes and 5-[(aminoalkyl)thio]-5H-[1]benzopyrano[2,3-b]pyridines, J. Med. Chem. 24 (1981) 1010-1013;(h) M. Venkati, G.L.D. Krupadanam, A facile synthesis of ethyl-2-methyl-5-aryl-5H-chromeno[3,4-c]pyridine-1-carboxylates, Synth. Commun. 31 (2001) 2589-2598. |
| [6] | M. Curini, G. Cravotto, F. Epifano, G. Giannone, Chemistry and biological activity of natural and synthetic prenyloxycoumarins, Curr. Med. Chem. 13 (2006) 199-222. |
| [7] | (a) A.M. El-Agrody, M.H. El-Hakim, M.S. Abd El-Latif, et al., Synthesis of pyrano[2,3-d]pyrimidine and pyrano[3,2-e][1,2,4]triazolo[2,3-c]pyrimidine derivatives with promising antibacterial activities, Acta Pharm. 50 (2000) 111-120;(b) M.C. Yimdjo, A.G. Azebaze, A.E. Nkengfack, et al., Antimicrobial and cytotoxic agents from calophyllum inophyllum, Phytochemistry 65 (2004) 2789-2795;(c) Z.Q. Xu, K. Pupek, W.J. Suling, L. Enache, M.T. Flavin, Pyranocoumarin, a novel anti-TB pharmacophore: synthesis and biological evaluation against Mycobacterium tuberculosis, Bioorg. Med. Chem 14 (2006) 4610-4626;(d) V. Jeso, K.C. Nicolaou, Total synthesis of tovophyllin B, Tetrahedron Lett. 50 (2009) 1161-1163;(e) L. Alvey, S. Prado, B. Saint-Joanis, et al., Diversity-oriented synthesis of furo[3,2-f]chromanes with antimycobacterial activity, Eur. J. Med. Chem. 44 (2009) 2497-2505. |
| [8] | (a) L. Alvey, S. Prado, V. Huteau, et al., A new synthetic access to furo[3,2-f]chromene analogues of an antimycobacterial, Bioorg. Med. Chem. 16 (2008) 8264-8272;(b) T. Symeonidis, M. Chamilos, D.J. Hadjipavlou-Litina, M. Kallitsakis, K.E. Litinas, Synthesis of hydroxycoumarins and hydroxybenzo[f]-or [h]coumarins as lipid peroxidation inhibitors, Bioorg. Med. Chem. Lett. 19 (2009) 1139-1142. |
| [9] | T. Narender, S. Gupta, A convenient and biogenetic type synthesis of few naturally occurring chromeno dihydrochalcones and their in vitro antileishmanial activity, Bioorg. Med. Chem. Lett. 14 (2004) 3913-3916. |
| [10] | (a) S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Pyran copolymer as an effective adjuvant to chemotherapy against a murine leukemia and solid tumor, Cancer Res. 35 (1975) 3750-3754;(b) A. Rampa, A. Bisi, F. Belluti, et al., Homopterocarpanes as bridged triarylethylene analogues: synthesis and antagonistic effects in human MCF-7 breast cancer cells, Farmaco 60 (2005) 135-147;(c) Q.B. Han, N.Y. Yang, H.L. Tian, et al., Xanthones with growth inhibition against HeLa cells from Garcinia xipshuanbannaensis, Phytochemistry 69 (2008) 2187-2192;(d) J.L. Wang, D.X. Liu, Z.J. Zhang, et al., Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells, Proc. Natl. Acad. Sci. U.S.A. 97 (2000) 7124-7129. |
| [11] | V.K. Tandon, M. Vaish, S. Jain, D.S. Bhakuni, R.C. Srimal, Synthesis, carbon-13 NMR and hypotensive action of 2,3-dihydro-2,2-dimethyl-4H-naphtho[1,2-b]pyran-4-one, Indian J. Pharm. Sci. 53 (1991) 22-23. |
| [12] | M. Brunavs, C. P. Dell, P. T.Gallagher, W. M. Owton, C. W. Smith. 4H-naphtho[1,2-b]pyran derivatives as antiproliferative agents. Eur. Pat. Appl. EP 557075 A1 19930825, (1993). |
| [13] | M. Longobardi, A. Bargagna, E. Mariani, et al., 2H-[1]benzothiepino[5,4-b]pyran derivatives with local anesthetic and antiarrhythmic activities, Farmaco 45 (1990) 399-404. |
| [14] | (a) K. Gorlitzer, A. Dehre, E. Engler, 2-(1H-Tetrazol-5-yl)-4,5-dihydroindeno[1,2-b]pyran-4-one, Arch. Pharm. 316 (1983) 264-270;(b) P. Coudert, J.M. Couquelet, J. Bastide, Y. Marion, J. Fialip, Synthesis and antiallergic properties of some N-arylnitrones having a furopyran structure, Ann. Pharm. Fr. 46 (1988) 91-96. |
| [15] | F. Eiden, F. Denk, Synthesis and CNS-activity of pyran derivatives: 6,8-dioxabicyclo[3.2.1]octanes, Arch. Pharm. 324 (1991) 353-354. |
| [16] | C. Bruhlmann, F. Ooms, P. Carrupt, et al., Coumarins derivatives as dual inhibitors of acetylcholinesterase and monoamine oxidase, J. Med. Chem. 44 (2001) 3195-3198. |
| [17] | S.R. Kesten, T.G. Heffner, S.J. Johnson, et al., Design, synthesis, and evaluation of chromen-2-ones as potent and selective human dopamine D4 antagonists, J. Med. Chem. 42 (1999) 3718-3725. |
| [18] | (a) M.A. Kulkarni, K.S. Pandit, U.V. Desai, U.P. Lad, P.P. Wadgaonkar, Diethylamine: a smart organocatalyst in eco-safe and diastereoselective synthesis of medicinally privileged 2-amino-4H-chromenes at ambient temperature, C. R. Chimie 16 (2013) 689-695;(b) A.K. Gupta, K. Kumari, N. Singh, D.S. Raghuvanshi, K.N. Singh, An eco-safe approach to benzopyranopyrimidines and 4H-chromenes in ionic liquid at room temperature, Tetrahedron Lett. 53 (2012) 650-653;(c) J.M. Doshi, D. Tian, C. Xing, Structure activity relationship studies of ethyl 2-amino-6-bromo-4-(1-cyano-2-ethoxy-2-oxoethyl)-4H-chromene-3-carboxylate (HA 14-1), an antagonist for antiapoptotic Bcl-2 proteins to overcome drug resistance in cancer, J. Med. Chem. 49 (2006) 7731-7739;(d) D. Greé, S. Vorin, V.L. Manthati, et al., The synthesis of new, selected analogues of the pro-apoptotic and anticancer molecule HA 14-1, Tetrahedron Lett. 49 (2008) 3276-3278;(e) P. Jayashree, G. Shanthi, P.T. Perumal, Indium trichloride catalyzed one-pot synthesis of new (2-amino-3-cyano-4H-chromen-4-yl) phosphonic acid diethyl ester, Synlett (2009) 917-920. |
| [19] | J. Volmajer, R. Toplak, I. Lebanb, A.M.L. Marechal, Synthesis of new iminocoumarins and their transformations into N-chloro and hydrazono compounds, Tetrahedron 61 (2005) 7012-7021. |
| [20] | M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 09, Revision C.02, Gaussian, Inc., Wallingford CT, Wallingford, 2009. |
| [21] | A.D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys. 98 (1993) 5648-5652. |
| [22] | (a) R. Krishnan, J.S. Binkley, R. Seeger, J.A. Pople, Self-consistent molecular orbital methods, XX. A basis set for correlated wave functions, J. Chem. Phys. 72 (1980) 650-654;(b) A.D. McLean, G.S. Chandler, Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11-18, J. Chem. Phys. 72 (1980) 5639-5648;(c) T. Clark, J. Chandrasekhar, G.W. Spitznagel, P. von Raye Schleyer, Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21 + G basis set for first-row elements, lithium to fluorine, J. Comp. Chem. 4 (1983) 294-301. |
| [23] | (a) B. Menucci, J. Tomassi, Continuum solvation models: a new approach to the problem of solute's charge distribution and cavity boundaries, J. Chem. Phys. 106 (1997) 5151-5158;(b) M. Cossi, V. Barone, B. Menucci, J. Tomassi, Ab initio study of ionic solutions by a polarizable continuum dielectric model, Chem. Phys. Lett. 286 (1998) 253-260. |