b Department of Chemistry, University of Education, Lahore 54770, Pakistan;
c Department of Chemistry, Michigan State University, E. Lansing, MI 48824, USA
Corrole is the prototypical example of a ring-contracted porphyrin in which one of the meso-carbon atoms is substituted with a bipyrrolic junction [1]. The discoveries of the facile syntheses of meso-triarylcorroles by Paolesse [2] and Gross [3], followed by a series of synthetic improvements by Gryko [4] and others [5-7] have revolutionized the field of corrole chemistry. Innovations in synthetic methodologies have made corroles readily affordable for studies in catalysis [8, 9],optical properties [10],photophysics [11],and biology [12-14]. These studies have also enabled the comparison of properties of corroles with the other pyrrole-aldehyde condensation products [15-17].
Among various types of corroles,the superstructured bispicket-fence corroles derived from 2,6-dibromobenzaldehyde may serve as excellent non-natural porphyrinoids for use in catalytic studies,coordination chemistry and heme-protein analogues [18, 19]. These brominated corroles also offer an easy approach towards bis-pocket bulky corroles via functionalization by Suzuki cross-coupling reaction [18-20]. Importantly,metallocorroles protected by bulky groups on the ortho-position of the meso-phenyl substituents may potentially escape from falling into the thermodynamic sink "µ-oxo and µ-peroxo bridging complex" when exposed to dioxygen or oxene species and thus behave very similar to the biological heme moiety encumbered in the protein pocket [21]. This feature is also suitable for their use as an excellent mechanistic probe during oxygenation reactions [22].
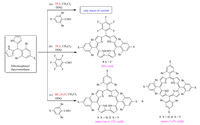
So far,various strategies such as the acid-catalyzed pyrrolealdehyde condensation under solvent-free conditions [3],modified Rothemund reaction [2],condensation between dipyrromethane [4] or higher oligopyrroles [5, 6] and aldehyde,as well as templated reaction of 2,2′-bisdipyrrins with manganese [7] have been adapted for corrole synthesis. Many single- and double-sided sterically encumbered corroles containing nitro [23],methyl [4, 24, 25],phenyl [26],or small electronegative chloro- or fluorosubstituents [3, 27] on the ortho-positions of meso-phenyl have been reported by utilizing the above methods. The absence of a single method for various corroles indicates that the synthesis of A3-type corroles is largely dependent on the type of aldehyde used. For example,a solvent-free method has been employed for the synthesis of highly electron-poor corroles,and modified Rothemund reaction,although suitable for relatively a broad range of electron-poor and electron-rich corroles,failed to give corrole in the case of highly electron-rich aldehydes such as 4-dimethylaminobenzaldehyde [28]. The synthesis of sterically encumbered A3-type corroles originating from ortho,ortho'-dibromo-substituted benzaldehydes (Scheme 1) has been reported inaccessible by routine methods [29]. The only report that appeared more recently is the trifluoroacetic acid (TFA) catalyzed synthesis of tris(2,6-dibromophenyl)corrole (Br6Cor 1,Scheme 1) via direct condensation of 2,6-dibromobenzaldehyde and pyrrole in 5% yield, while the whole synthesis should be performed under red light irradiation [20]. In connection with our previous work focused on the synthesis of selective peripherally decorated porphyrinoids [26, 27, 30], we here wish to report an improved synthesis of A3-type sterically encumbered multibrominated corroles (Scheme 1) by condensation of dipyrromethane and aldehyde using boron trifluoride dietherate (BF3.Et2O) as a catalyst. This method is found to be more efficient in terms of yield (up to 12%),ease of handling,and reproducibility.
|
Download:
|
| Scheme 1.Synthesis of sterically encumbered multibrominated corroles. | |
Typical procedure for method A: Dibromobenzaldehyde (1 mmol) was added to excess pyrrole (120 mL approx.) along with catalytic amounts of TFA (16 µL,0.21 mmol) or BF3.Et2O (0.1 mL,46.8%-47.8%,d = 1.120-1.140 g/mL). After stirring the solution mixture for 30 min,the excess of pyrrole was removed by distillation. After cooling,the crude solid was purified on silica gel column using dichloromethane as eluent. The purified dibromophenyl dipyrromethane was dissolved in dry dichloromethane (50 mL) along with the aldehyde in a 2:1 ratio. TFA (16 µL, 0.21 mmol) or BF3.Et2O (500 mL) was subsequently added. The mixture was stirred for 3-4 h. After quenching with triethylamine (Et3N),DDQ (227 mg,1 mmol) was added and stirring was continued for another 1 h. Excess solvent was removed under vacuum and the crude product was purified on silica gel column using hexane/diethyl ether (v/v) (20:1 for 1,10:1 for 2,and 30:1 for 5). The characterization data for each compound is given below:
5,10,15-Tris(2,6-dibromophenyl) corrole 1: UV-vis (CH2Cl2, nm): λmax418,516,567,608;1H NMR (400 MHz,CDCl3): δ 8.93 (d, 2H,J = 3.7 Hz),8.49 (d,2H,J = 4.4 Hz),8.38-8.31 (dd,4H,J = 10.8, 3.9 Hz),7.99 (dd,6H,J = 8.1,3.1 Hz),7.50-7.44 (m,3H); 13C NMR(100 MHz,CDCl3): δ 142.5,142.6,131.8,131.66,131.62,131.1, 130.9,129.4,129.2,125.5,117.1,116.1,115.97,115.96,109.9; MS (MALDI-TOF): Found: 999.540 ([M]+),Calcd.: 999.67. The characterization data concur with the published data [20].
5,10,15-Tris(2,6-dibromo-4-fluorophenyl) corrole 2: UV-vis (CH2Cl2,nm): λmax414,519,566,608; 1H NMR (400 MHz,CDCl3): δ 8.95 (d,2H,J = 3.7 Hz),8.51 (d,2H,J = 4.3 Hz),8.38-8.34 (dd,4H, J = 8.4,4.3 Hz),7.80-7.77 (dd,6H,J = 7.8,3.3 Hz),-2.49 (s,3H); 19F NMR (376 MHz,CDCl3): δ -109.88 (s,2F),-110.32 (s,1F); 13C NMR(100 MHz,CDCl3): δ 163.4,160.9,136.9,134.2,130.6,129.9,129.7, 129.1,127.9,126.9,125.5,119.5,119.3,119.1,116.1,109.1; MS(ESI): 1054.9 (MH+); HRMS: m/z 1054.6516 (MH+,C37H18Br6F3N4 requires 1054.6516).
5,15-(2,6-Dibromo-4-fuorophenyl)-10-pentafluorophenyl corrole 5: UV-vis (CH2Cl2,nm): λmax410,516,563,603; 1H NMR(400 MHz,CDCl3): δ 8.99 (d,2H,J = 4.2 Hz),8.56 (d,2H,J = 4.7 Hz), 8.47 (d,2H,J = 4.7 Hz),8.40 (d,2H,J = 4.1 Hz),7.80 (d,4H, J = 7.8 Hz),-2.67 (s,3H);19F NMR (376 MHz,CDCl3): δ -109.58 (s, 2F),-136.95 (dd,2F,J = 24.3,8.4 Hz),-153.40 (t,1F,J = 20.9 Hz), -162.31 (dt,2F,J = 24.3,8.4 Hz);13C NMR (100 MHz,CDCl3): δ 163.4,160.9,151.5,149.9,143.0,136.6,133.9,133.8,142.9,141.9, 141.6,129.6,128.0,127.9,125.7,123.9,120.4,119.6,119.3,116.1, 115.3,109.0,107.9; MS (MALDI-TOF): 967.7 ([M]+); HRMS: m/z 968.7946 (MH+,C37H16Br4F7N4 requires 968.7991).
5,10,15,20-Tetrakis(2,6-dibromo-4-fluorophenyl) porphyrin 4: Porphyrin 4 was isolated as side product during the synthesis of corrole 2. UV-vis (CH2Cl2,nm): λmax421,517,556,598,661; 1H NMR (400 MHz,CDCl3): δ 8.65 (s,8H),7.82 (d,8H,J = 7.8 Hz), -2.49 (s,3H);19F NMR (376 MHz,CDCl3): δ -109.51 (s,4F); 13C NMR (100 MHz,CDCl3): δ 165.1,164.0,159.7,153.1,152.6,149.7, 143.0,143.7,143.9,139.7,139.6,139.1,136.4,136.5,132.7,135.2, 131.9,129.9,129.6,127.6,127.2,120.0,119.7,119.4; MS (MALDITOF): 1317.303 ([M]+) (clusters of peaks due to Br isotope distribution); HRMS: m/z 1318.4957 (MH+,C44H19Br8F4N4 requires 1318.4957).
5,10,15,20-Tetrakis(2,6-dibromophenyl) porphyrin 3: Porphyrin 3 [31] was isolated as side product during the synthesis of corrole 1. UV-vis (CH2Cl2,nm): λmax422,520,532,600,649; MS (MALDI-TOF): Found: 1245.379 ([M]+),Calcd.: 1245.52. 3. Results and discussion
In fact,we have been attempting the synthesis of A3-type corrole from ortho,ortho′-dibromo-substituted benzaldehyde for a long time. The traditional one-pot methods such as the solventfree method [3] and the modified Rothemund reaction [2, 24, 25, 28] did not yield the desired corrole starting from ortho-dibromobenzaldehyde under the exactly reported conditions. We then turned to the TFA catalyzed dipyrromethane-aldehyde condensation method [4] because of its general application for the synthesis of a wide variety of corroles in good yields. To our surprise,only traces of hexabrominated corroles (Br6Cor 1 and F3Br6Cor 2) could be isolated via TFA catalyzed condensation of 2,6-dibromobenzaldehyde and corresponding dipyrromethane (Scheme 1,path a). In contrast,TFA catalyzed condensation of 5-(2,6-dibromo-4-fluorophenyl)-dipyrromethane with pentafluorobenzaldehyde gave trans-A2B corrole (F7Br4Cor 5) in 20% yield (Scheme 1,path b). This implied that TFA may not be the suitable catalyst for preparing A3-type sterically encumbered multibrominated corroles by this method. This may probably be attributed to the relatively moderate catalytic activity of TFA that fails to enable macrocyclization of the corrole precursor effectively due to steric crowding [32].
In comparison with the TFA catalyzed dipyrromethane procedure,our decision to follow the one-pot method developed by Broring [20] proved to be more useful,and the hexabrominated corrole (Br6Cor 1) was obtained in 4.5% yield. However,it was found that this method is very sensitive to the conditions employed,and the yield may drop sometimes unexpectedly, giving the same corrole (Br6Cor 1) in as low as 1% yield. We also experienced that this method is not convenient to handle owing to its application on small scale and the absence of any other solvent. A similar phenomenon was observed for 2,6-dibromo-4-fluoro benzaldehyde,whose corresponding corrole,tris(2,6-dibromo-4-fluorophenyl)corrole (F3Br6Cor 2,Scheme 1) was obtained in the best yield of 5%. Our attempts to scale-up the same reaction and to improve the yield of corroles by employing this one-pot method failed.
We were directed to use BF3.Et2O as a catalyst in the synthesis of sterically encumbered multibrominated corroles mainly from the independent work of Dehaen [33] and Collman [32]. They employed two different strategies to synthesize ortho-disubstituted A3-type or trans-A2B corroles by using BF3.Et2O catalyst. One strategy is the condensation of dipyrromethane-dicarbinol and 2,2′-bipyrrole [32],and the other is the condensation of aryldipyrromethane and arylaldehyde [33]. These methods were proven efficient for the synthesis of corrole derivatives starting from relatively less-hindered aldehydes such as mesitaldehyde and 2,6-dichloro benzaldehyde. Inspired by their work,we switched to the use of BF3.Et2O as a catalyst in the condensation of 2,6-dibromophenyl-substituted dipyrromethane and 2,6-dibromobenzaldehyde for the synthesis of corresponding A3-type sterically encumbered multibrominated corroles. This turned out to be more surprising and the target corrole was obtained in a satisfactory yield of ~12% (Table 1 and Scheme 1,path c). All the synthesized multibrominated corroles are well characterized by UV-vis,mass and NMR (1H,19F and 13C) spectroscopic techniques, and the characterization data is summarized in experimental section. Fig. 1 shows the absorption spectra of synthesized multibrominated corroles (Br6Cor 1,F3Br6Cor 2,F7Br4Cor 5). The sharp Soret-band at about 400 nm and three set of Q-bands in the range of 510-610 nm are in accordance with the typical corroles’ spectra. The sharp signals in the 1H NMR spectra (Fig. 2) for bpyrrole protons in the range of δ 9.0-8.3 and phenyl protons in the region of δ 8.1-7.4 also corroborate the existence of corrole macrocycle [3, 20].
| Table 1 Yields of A3 and trans-A2B multibrominated corroles by different methods. |

|
Download:
|
| Fig. 1.UV-vis spectra (in CH2Cl2) of multibrominated corroles Br6Cor 1,F3Br6Cor 2,and F7Br4Cor 5. | |

|
Download:
|
| Fig. 2.Partial 1H NMR spectra (in CDCl3) of Br6Cor 1 and F3Br6Cor 2 showing the aromatic and b-pyrrole protons. | |
While dipyrromethane may be separated from the other impurities by column chromatography on silica gel,it was observed that treating the crude mixture containing the dipyrromethane with the aldehyde also gave the corresponding A3-type corrole in a comparable yield. Although dipyrromethane may be obtained via TFA or BF3.Et2O catalyzed condensation of 2,6-dibromo-benzaldehyde and pyrrole,but its further condensation with the aldehyde to generate the sterically encumbered multibrominated corrole should be catalyzed by BF3.Et2O. It must be noted that minor amounts (1-2%) of the corresponding symmetrical porphyrin derivatives were also isolated during the corrole synthesis by this method. This may be attributed to BF3.Et2O catalyst that favours the formation of porphyrin due to its ability to coordinate with the pyrrole nitrogen atoms during pyrrolealdehyde condensation. The preferential synthesis of corroles as major products by BF3.Et2O catalyst in the current reaction system is controlled by 2:1 ratio between dipyrromethane and aldehyde. This situation can be changed,and even reversed,by changing the ratio between dipyrromethane and aldehyde.
The yields of 2,6-dibromo-substituted corroles obtained via BF3.Et2O catalyst are comparable with the other sterically hindered 2,6-dichloro-,2,6-difluoro- and 2,4,6-trimethylphenyl-substituted corrole derivatives obtained by various methods [24, 25, 32]. We noted that a smaller concentration of BF3.Et2O [2-3 mmol/L] is beneficial for the synthesis of multibrominated corroles. The yield of A3-type multibrominated corroles was observed to decrease sharply if the condensation of dipyrromethane and aldehyde is carried out for a shorter time (less than 1 h),in contrast with tris(2,6-dichlorophenyl)corrole where 30 min stirring was sufficient for 22% yield of the corrole [33]. It appeared that relatively longer reaction time (3-4 h) is crucial for the effective cyclization of multibrominated corroles precursors due to the steric crowding, similar to previous observations for other sterically encumbered corroles [32]. By comparing the above,it is evident that while TFA may be used for the synthesis of sterically encumbered multibrominated corroles by direct condensation of aldehyde and pyrrole in the absence of any other solvent and prolonged reaction time (16 h) under red light irradiation [16],BF3.Et2O is a better choice because of higher yields and shorter reaction time. 4. Conclusion
TheIn summary,we report an efficient method for the synthesis of meso-ortho,ortho′-dibromophenyl substituted A3-type corroles in good yields. We have also explored the efficiency of two widely used catalysts,TFA and BF3.Et2O,for the synthesis of sterically encumbered multibrominated corroles. The current study elaborates that sterically hindered multibrominated corroles are easily accessible in appreciable yields via BF3.Et2O catalyzed condensation of ortho-dibromobenzaldehyde and corresponding dipyrromethane. Further studies will be focused on the utilization of these sterically shielded superstructures for peripheral functionalization,and their subsequent applications in catalysis. Acknowledgment
We thankfully acknowledge financial support from National Natural Science Foundation of China (Nos. 21171057 and 21371059).
| [1] | S. Nardis, D. Monti, R. Paolesse, Novel aspects of corrole chemistry, Mini-Rev. Org. Chem. 2 (2005) 355-374. |
| [2] | R. Paolesse, L. Jaquinod, D.J. Nurco, et al., 5,10,15-Triphenylcorrole: a product from a modified Rothemund reaction,, Chem. Commun. (1999) 1307-1308. |
| [3] | Z. Gross, N. Galili, I. Saltsman, The first direct synthesis of corroles from pyrrole, Angew. Chem. Int. Ed. 38 (1999) 1427-1429. |
| [4] | D.T. Gryko, A simple, rational synthesis of meso-substituted A2B-corroles, Chem. Commun. (2000) 2243-2244. |
| [5] | R.P. Briñas, C. Brückner, Triarylcorroles by oxidative coupling of triaryltetrapyrranes, Synlett 3 (2001) 442-444. |
| [6] | J.W. Ka, C.H. Lee, Optimizing the synthesis of 5,10-disubstituted tripyrromethanes, Tetrahedron Lett. 41 (2000) 4609-4613. |
| [7] | M. Bröring, C. Hell, Manganese as a template: a new synthesis of corrole, Chem. Commun. (2001) 2336-2337. |
| [8] | H.Y. Liu, M.H.R. Mahmood, S.X. Qiu, C.K. Chang, Recent developments in manganese corroles, Coord. Chem. Rev. 257 (2013) 1306-1333. |
| [9] | N.C. Ng, M.H.R. Mahmood, H.Y. Liu, et al., Appended corrole manganese complexes: catalysis and axial-ligand effect, Chin. Chem. Lett. 25 (2014) 571-574. |
| [10] | X. Ying, X.Y. Long, M.H.R. Mahmood, et al., Second order nonlinear optical properties of corroles: experimental and theoretical investigations, J. Porphy. Phthalocy. 16 (2012) 1276-1284. |
| [11] | W.L. Shao, H. Wang, S. He, et al., Photophysical properties and singlet oxygen generation of three sets of halogenated corroles, J. Phys. Chem. B 116 (2012) 14228-14234. |
| [12] | L. Shi, H.Y. Liu, L.P. Si, et al., The heavy atom effect on photocleavage of DNA by mono-hydroxyl halogentaed corroles, Chin. Chem. Lett. 21 (2010) 373-375. |
| [13] | J. Lu, H.Y. Liu, L. Shi, et al., DNA cleavage mediated by water-soluble manganese corrole, Chin. Chem. Lett. 22 (2011) 101-104. |
| [14] | Y. Zhang, Q. Wang, J.Y. Wen, et al., DNA binding and oxidative cleavage by a watersoluble carboxyl manganese(III) corrole, Chin. J. Chem. 31 (2013) 1321-1328. |
| [15] | Y. Xie, J.P. Hill, A.L. Schumacher, et al., Tautomerism in novel oxocorrologens, Chem. Eur. J. 13 (2007) 9824-9833. |
| [16] | Y.S. Xie, K. Yamaguchi, M. Toganoh, et al., Triply N-confused hexaphyrins: nearinfrared luminescent dyes with a triangular shape, Angew. Chem. Int. Ed. 48 (2009) 5496-5499. |
| [17] | Y.S. Xie, P.C. Wei, X. Li, et al., Macrocycle contraction and expansion of a dihydrosapphyrin isomer, J. Am. Chem. Soc. 135 (2013) 19119-19122. |
| [18] | M. Bröring, C. Milsmann, S. Ruck, S. Köhler, Bis-picket-fence corroles, J. Organo-met. Chem. 694 (2009) 1011-1015. |
| [19] | I. Nigel-Etinger, A. Mahammed, Z. Gross, Covalent versus non-covalent (biocatalytic) approaches for enantioselective sulfoxidation catalyzed by corrole metal complexes, Catal. Sci. Technol. 1 (2011) 578-581. |
| [20] | M. Bröring, M. Funk, C. Milsmann, Investigating different strategies towards the preparation of chiral and achiral bis-pocket fence corroles, J. Porphy. Phthalocy. 13 (2009) 107-113. |
| [21] | N. Bag, S.S. Chern, S.M. Peng, C.K. Chang, Bis-pocket porphyrins without mesosubstituents: tetramethyltetra(2,4,6-triisopropylphenyl)porphyrin I and tetramethyltetraterphenylporphyrin I, Tetrahedron Lett. 36 (1995) 6409-6412. |
| [22] | B. Meunier, Metalloporphyrins as versatile catalysts for oxidation reactions and oxidative DNA cleavage, Chem. Rev. 92 (1992) 1411-1456. |
| [23] | E. Rose, B. Andrioletti, Synthesis of the first superstructured chiral corrole, J. Chem. Soc. Perkin Trans. 1 (2002) 715-716. |
| [24] | D.T. Gryko, B. Koszarna, Refined methods for the synthesis of meso-substituted A3-and trans-A2B-corroles, Org. Biomol. Chem. 1 (2003) 350-357. |
| [25] | B. Koszarna, D.T. Gryko, Efficient synthesis of meso-substituted corroles in a H2OMeOH mixture, J. Org. Chem. 71 (2006) 3707-3717. |
| [26] | H.Y. Liu, F. Yam, Y.T. Xie, X.Y. Li, C.K. Chang, A bulky bis-pocket manganese(V)-oxo corrole complex: observation of oxygen atom transfer between triply bonded MnV≡O and alkene, J. Am. Chem. Soc. 131 (2009) 12890-12891. |
| [27] | H.Y. Liu, T.S. Lai, L.L. Yeung, C.K. Chang, First synthesis of perfluorinated corrole and its MnO complex, Org. Lett. 5 (2003) 617-620. |
| [28] | M.H.R. Mahmood, H.Y. Liu, H.H. Wang, Y.Y. Jiang, C.K. Chang, Unexpected one-pot synthesis of A3-type unsymmetrical porphyrin, Tetrahedron Lett. 54 (2013) 5853-5856. |
| [29] | A. Kumar, I. Goldberg, M. Botoshansky, et al., Oxygen atom transfer reactions from isolated (oxo)manganese(V)corroles to sulfides, J. Am. Chem. Soc. 132 (2010) 15233-15245. |
| [30] | C.K. Chang, C.Y. Yeh, T.S. Lai, Synthesis of sterically encumbered porphyrins as catalyst for shape-selective oxidations, Macromol. Symp. 156 (2000) 117-124. |
| [31] | D. Ostović, T.C. Bruice, Transition-state geometry in epoxidation by iron-oxo porphyrin at the compound I oxidation level. Epoxidation of alkenes catalyzed by a sterically hindered (meso-tetrakis(2,6-dibromophenyl)porphyrinato)iron(III) chloride, J. Am. Chem. Soc. 110 (1988) 6906-6908. |
| [32] | R. Decréau, J.P. Collman, Corrole synthesis by dipyrromethane-dicarbinol and 2,2'-bipyrrole condensation, Tetrahedron Lett. 44 (2003) 3323-3327. |
| [33] | C.V. Asokan, S. Smeets, W. Dehaen, Sterically encumbered triarylcorroles from aryldipyrromethanes and aromatic aldehydes, Tetrahedron Lett. 42 (2001) 4483-4485. |