Multicomponent reactions (MCR) combined with the application of task specific ionic liquids (TSIL) are valuable tools for the environmentally friendly preparation of structurally diverse chemical libraries of drug-like heterocyclic compounds [7]. Tetrahydrobenzo[a]xanthene derivatives act as anti-microbial [8],anti-malarial [9],and cytotoxic agents [10]. Furthermore, some of these compounds have been used in photodynamic therapy [11],dyes [12],pH-sensitive fluorescent materials for visualization of biomolecules [13],and laser technology [14]. 12-Aryl-8,9,10,12-tetrahydrobenzo[a]xanthenes can be prepared by multicomponent condensation of 2-naphthols,aldehydes,and cyclic 1,3-dicarbonyl compounds in the presence of various acid catalysts [15]. Some of the reported methods have limitations such as longer reaction time,harsher reaction conditions,expensive catalysts,and generation of large amounts of side products. In view of our interest in the development of new syntheses of various heterocycles [16],we investigated the synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives by one pot three-component condensation of naphthols,aldehydes, and cyclic 1,3-dicarbonyl compounds in task specific acidic ionic liquid [NMP]H2PO4. 2. Experimental
All chemicals were purchased from Sigma-Aldrich,Spectrochem and were used as received. F254 precoated aluminium plates with silica gel 60 from Merck were used to monitor reaction progress. IR (KBr) spectra were recorded on a Perkin Elmer FTIR spectrophotometer,and the values are expressed as vmax. The NMR ( 1H and 13C) spectra were recorded on a Jeol JNM ECX-400P at 400 MHz and 100 MHz,respectively. The chemical shift values are recorded on d scale and the coupling constants (J) are in Hertz. Ionic liquid [NMP]H2PO4 was prepared according to the literature procedure [6]. 2.1. General procedure for the synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives ((1a)-1y) A mixture of 2,7-dihydroxynaphthalene/2-naphthol/2,6-dihydroxynaphthalene (1.0 mmol),substituted aromatic aldehydes (1.0 mmol),dimedone/cyclohexane-1,3-dione (1.1 mmol) and task specific ionic liquid [NMP]H2PO4(20 mol%) was placed in a 50 mL round-bottomed flask mounted over a magnetic stirrer. The reaction mixture was stirred at 80 ℃ for the appropriate time as mentioned in Table 3. The progress of the reaction was monitored by TLC using ethyl acetate: petroleum ether (20:80,v/v) as the eluent. After completion of the reaction as indicated by TLC,water (10 mL) was added to the reaction mixture. The precipitate formed was collected by vacuum filtration and washed with water. The product was recrystallized from ethanol to give the pure product ((1a)-1y) in high yield. The products were characterized by mp,IR, NMR and mass spectra.
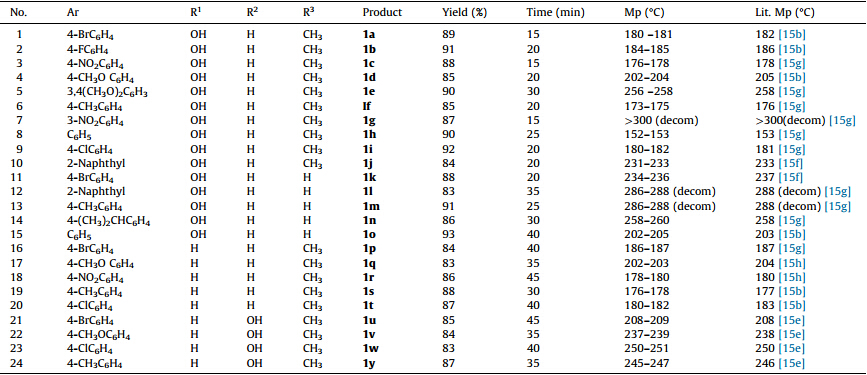
| Table 3 Synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives (1a-1y) |
12-(4-Bromophenyl)-2-hydroxy-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one ((1a)) [15b]: White solid; mp: 180- 181 ℃; 1H NMR (400 MHz,DMSO-d6): δ 9.89 (s,1H,OH),7.77-7.74 (m,2H,ArH),7.40 (d,2H,J = 6.9 Hz,ArH),7.19 (d,4H,J = 8.7 Hz, ArH),6.98 (d,1H,J = 8.7 Hz,ArH),5.32 (s,1H,ArCH),2.67 and 2.56 (AB system,2H,J = 17.7 Hz,CHaHb),2.34 (d,1H,J = 16.2 Hz, CHaCO),2.13 (d,1H,J = 16.2 Hz,CHbCO),1.05 (s,3H,CH3),0.86 (s, 3H,CH3); 13C NMR (100 MHz,DMSO-d6): δ 195.92,164.02,156.52, 147.63,144.00,132.32,131.00,130.28,129.01,125.48,119.26, 117.19,114.74,113.51,112.75,105.06,50.04,40.34,33.79,31.85, 28.78,26.21; IR (KBr,cm-1 ): vmax3328,2959,1626,1597,1380, 1225; MS (ESI) [M+] calcd. for C25H21BrO3: 448,found: 449 [M+ +H], 451 [M+ +H+2].
12-(4-Bromophenyl)-2-hydroxy-8,9,10,12-tetrahydrobenzo[a] xanthen-11-one (1k) [15f]: White solid; mp: 234-236 ℃; 1H NMR (400 MHz,DMSO-d6): δ 9.89 (s,1H,OH),7.73-7.78 (m,2H,ArH), 7.41 (d,2H,J = 8.4 Hz,ArH),7.13-7.21 (m,4H,ArH),6.95-6.99 (m, 1H,ArH),5.35 (s,1H,ArCH),2.73-2.69 (m,2H,CH2),2.37-2.26 (m, 2H,CH2),1.86-1.80 (m,2H,CH2); 13C NMR (100 MHz,DMSO-d6): δ 196.19,165.90,156.49,147.65,144.27,132.33,131.06,130.32, 130.28,128.99,125.44,119.28,117.16,114.70,113.96,113.46, 105.01,36.36,33.70,26.90,19.88; IR (KBr,cm-1 ): vmax3326,2944, 1626,1597,1379,1231,1197; MS (ESI) [M+] calcd. for C23H17BrO3: 421,found: 422 [M+ +H],424 [M+ +H+2].
12-(4-Bromophenyl)-9,9-dimethyl-8,9,10,12-tetrahydrobenzo [a]xanthen-11-one (1p) [15g]: White solid; mp: 186-187 ℃; 1 H NMR (400 MHz,DMSO-d6): δ 7.89 (d,1H,J = 8.3 Hz,ArH),7.80- 7.76 (m,2H,ArH),7.45-7.38 (m,2H,ArH),7.33-7.23 (m,3H,ArH), 7.23-7.20 (m,2H,ArH),5.66 (s,1H,ArCH),2.60 and 2.50 (AB system,2H,J = 17.60 Hz,CHaHb),2.24 (d,1H,J = 16.2 Hz,CHaCO), 2.12 (d,1H,J = 16.2 Hz,CHbCO),1.12 (s,3H,CH3),0.96 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6): δ 196.75,164.0,147.85,143.7, 131.45,131.22,130.10,129.12,128.56,127.20,125.23,123.48, 120.55,117.25,116.89,113.52,50.23,41.23,34.69,32.87,29.78, 26.98; IR (KBr,cm-1 ): vmax2955,1652,1596,1373,1228,1160; MS (ESI) [M+] calcd. for C25H21BrO2: 432,found: 433 [M+ +H],435 [M+ +H+2].
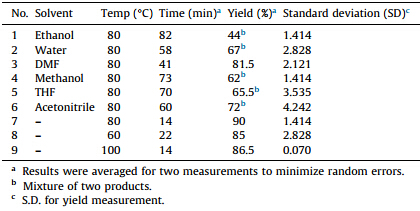
12-(4-Bromophenyl)-3-hydroxy-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one (1u) [15e]: White solid; mp: 208- 209 ℃; 1H NMR (400 MHz,DMSO-d6): δ 9.79 (s,1H,OH),7.82 (d, 1H,J = 9.5 Hz,ArH),7.67 (d,1H,J = 9.5 Hz,ArH),7.37-7.33 (m,3H, ArH),7.20 (d,2H,J = 8.04 Hz,ArH),7.13-7.10 (m,1H,ArH),7.05- 7.02 (m,1H,ArH),5.48 (s,1H,ArCH),2.64 and 2.53 (AB system,2H, J = 17.45 Hz,CHaHb),2.30 (d,1H,J = 16.2 Hz,CHaCO),2.10 (d,1H, J = 16.2 Hz,CHbCO),1.03 (s,3H,CH3),0.85 (s,3H,CH3); 13C NMR (100 MHz,DMSO-d6): δ 195.96,164.10,154.70,145.01,144.43, 132.70,131.02,130.66,130.30,127.48,124.78,124.51,119.40, 119.23,117.30,116.73,112.53,110.01,50.08,33.78,31.90,28.73, 28.60,26.50,26.20; IR (KBr,cm-1 ): vmax3340,2950,1614,1547, 1379,1220; MS (ESI) [M+] calcd. for C25H21BrO3: 448,found: 449 [M+ +H],451 [M+ +H+2]. 3. Results and discussion We present herein an efficient and environmentally benign protocol for the synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives by one pot condensation of 2-naphthols,aldehydes and cyclic 1,3-dicarbonyl compounds using task specific acidic ionic liquid [NMP]H2PO4 at 80 ℃. The most suitable reaction conditions for the proposed reaction were achieved by investigating the model reaction of 4-bromobenzaldehyde (1.0 mmol),2,7-dihydroxynaphthalene (1.0 mmol),and dimedone (1.1 mmol) under different reaction conditions as shown in Table 1. The best result was obtained when the reaction was carried at 80 ℃ in the absence of any solvent using 20 mol% of task specific ionic liquid NMP[H2PO4]. The reaction was complete in 14 min and yielded 90% of 12-(4-bromophenyl)-2-hydroxy-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one ((1a)) after a simple workup (Table 1,entry 7). Reactions attempted in solvents such as ethanol,water,DMF,methanol,THF and acetonitrile in the presence of ionic liquid [NMP]H2PO4 (20 mol%) as the catalyst required longer reaction time and resulted in inferior yields of the product (1a (Table 1,entries 1-6). Reactions repeated at 60 ℃ required longer times while reactions attempted at 100 ℃ required the same reaction times (Table 1, entries 8 and 9). Thus,ionic liquid [NMP]H2PO4with dual roles of solvent and catalyst was found to be most suitable for this threecomponent reaction for the synthesis of benzo[a]xanthene derivatives.
| Table 1 Effect of solvent and temperature on the reaction of 2,7-dihydroxynaphthlaene, 4-bromobenzaldehyde and dimedone. |
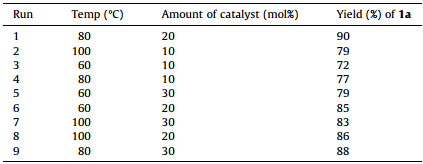
We study the comprehensive effect of temperature and amount of acidic ionic liquid [NMP]H2PO4on the yield of 1a using factorial analysis. Factorial analysis was carried out by considering two experimental factors with three levels,i.e. temperature (with levels 60 ℃,80 ℃,100 ℃) and amount of ionic liquid (with levels 10 mol%,20 mol%,30 mol%). The model reaction for the synthesis of 1a was performed under different conditions as obtained by fully designed factorial array for these factors (Table 2).
| Table 2 Full designed factorial array for the study of temperature and amount of catalyst over yield of 1a. |
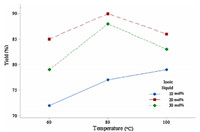
The factorial analysis was performed using Minitab 17 software, and the results of factorial analysis are shown in Fig. 1. From Fig. 1 it can be concluded that the combination of two experimental parameters,i.e.,temperature of 80 ℃ and ionic liquid NMP[H2PO4] (20 mol%),gave the best yield of compound 1a (90%). Thus,the three-component reaction of 4-bromobenzaldehyde (1.0 mmol), 2,7-dihydroxynaphthalene (1.0 mmol),and dimedone (1.1 mmol) in the presence of NMP[H2PO4] (20 mol%) at 80 ℃ was chosen as the most suitable set reaction conditions for this reaction,as these gave the product in the highest yield (90%) in the shortest reaction time.
|
Download:
|
| Fig. 1. Results of factorial analysis for the effect temperature and amount of ionic liquid over yield of 1a. | |
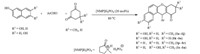
Subsequently,reactions of variously substituted aromatic aldehydes with 2,7-dihydroxynaphthalene and dimedone were carried out under these most suitable reaction conditions. The reactions proceeded smoothly for different aromatic aldehydes to afford the corresponding 12-aryl-2-hydroxy-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives in high yields (1b-1j, Table 3). The protocol was further extended by exploring the cyclocondensation of aromatic aldehydes and 2,7-dihydroxynaphthalene with cyclohexane-1,3-dione. The reaction of 4-bromobenzaldehyde (1.0 mmol),2,7-dihydroxynaphthalene (1.0 mmol),and cyclohexane-1,3-dione (1.1 mmol) in the presence of ionic liquid [NMP]H2PO4(20 mol%) at 80 ℃ yielded 88% of 12-(4-bromophenyl)-2-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one (1k) after 20 min. Other substituted benzaldehyde derivatives also underwent successful condensation,giving excellent yields of corresponding 12-aryl-2-hydroxy-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives (1l-1o,Table 3).
The scope of the three-component reaction was further investigated with other naphthols. It was observed that 2-naphthol (1.0 mmol) also underwent reactions with dimedone (1.1 mmol) and substituted aromatic aldehydes (1.0 mmol) in the presence of [NMP]H2PO4(20 mol%) at 80 ℃ giving high yields of corresponding 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives in good yield (1p-1t,Table 3). Similarly,reaction of 2,6-dihydroxynaphthalene (1.0 mmol) with dimedone (1.1 mmol) and various aromatic aldehydes (1.0 mmol) under the above optimized reaction conditions proceeded smoothly to afford the corresponding 12-aryl-3-hydroxy-8,9,10,12-tetrahydrobenzo[a]xanthene-11-ones derivatives in high yields (1u-1y,Table 3). However,no xanthene derivatives were obtained when the reactions were attempted with sesamol,1-naphthol,1,5-dihydroxynaphthalene and 1,6-dihydroxynaphthalene. Also all our efforts to achieve biscondensation of 2,6- and 2,7-dihydroxynaphthalenes using two molar equivalents of aldehyde and active methylene compounds have also been unsuccessful under these conditions. The generalized reaction scheme is shown in Scheme 1.
|
Download:
|
| Scheme 1.Synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene-11-one derivatives in ionic liquid [NMP]H2PO4. | |
The recyclability of ionic liquid [NMP]H2PO4 was also investigated,as it is an important aspect of green chemistry. After completion of the reaction for synthesis of 1a,the reaction mixture was cooled to room temperature and water (5 mL) was added. The ionic liquid dissolved in water,and the solution was filtered to isolate the product. The ionic liquid was recovered by evaporating the water containing the ionic liquid,and the remaining viscous liquid was washed with CH2Cl2(5 mL) and dried under reduced pressure. The recovered ionic liquid was tested to study its catalytic activity in the subsequent runs. No appreciable loss in the yield of 1a was observed after four cycles,as 1a was obtained in 89%,88%,85%,and 81% yield after the first,second,third and fourth cycle,respectively. However,yield of 1a was reduced to 74% in the fifth cycle.
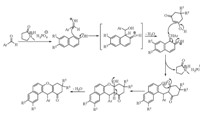
The plausible reaction mechanism is given in Scheme 2. The reaction proceeds through the in situ formation of ortho-quinone methide intermediate by the nucleophilic addition of 2-naphthol derivative to aldehyde in the presence of [NMP]H2PO4which is further attacked by cyclic 1,3-dicarbonyl compound followed by cyclization and elimination of water to yield the 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene derivatives.
|
Download:
|
| Scheme 2.Plausible reaction mechanism for synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthene derivatives in task specific ionic liquid [NMP]H2PO4 at 80 ℃ | |
| [1] | P. Tundo, P. Anastas, D.S. Black, et al., Synthetic pathways and processes in green chemistry. Introductory overview, Pure Appl. Chem. 72 (2000) 1207-1228. |
| [2] | D.J.C. Constable, C.J. Gonzalez, R.K. Henderson, Perspective on solvent use in the pharmaceutical industry, Org. Process Res. Dev. 11 (2007) 133-137. |
| [3] | D. Reinhardt, F. Ilgen, D. Kralisch, B. König, G. Kreisel, Evaluating the greenness of alternative reaction media, Green Chem. 10 (2008) 1170-1181. |
| [4] | (a) T. Welton, Room-temperature ionic liquids. Solvents for synthesis and catalysis, Chem. Rev. 99 (1999) 2071-2083; |
| [5] | M. Deetlefs, K.R. Seddon, Assessing the greenness of some typical laboratory ionic liquid preparations, Green Chem. 12 (2010) 17-30. |
| [6] | H. Guo, X. Li, J.L. Wang, X.H. Jin, X.F. Lin, Acidic ionic liquid [NMP]H2PO4 as dual solvent-catalyst for synthesis of β-alkoxyketones by the oxa-Michael addition reactions, Tetrahedron 66 (2010) 8300-8303. |
| [7] |
(a) A. Dömling, Recent developments in isocyanide based multicomponent reactions in applied chemistry, Chem. Rev. 106 (2006) 17-89; (b) C. Hulme, V. Gore, Multi-component reactions: emerging chemistry in drug discovery ‘from xylocain to crixivan', Curr. Med. Chem. 10 (2003) 51-80; (c) V. Nair, C. Rajesh, A.U. Vinod, et al., Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes, Acc. Chem. Res. 36 (2003) 899-907; (d) I. Ugi, B. Verner, A. Dmling, The chemistry of isocyanides, their multicomponent reactions and their Libraries, Molecules 8 (2003) 53-66; (e) J.P. Zhu, Recent developments in the isonitrile-based multicomponent synthesis of heterocycles, Eur. J. Org. Chem. (2003) 1133-1144;> (f) J. Chen, S.K. Spear, J.G. Huddleston, R.D. Rogers, Polyethylene glycol and solutions of polyethylene glycol as green reaction media, Green Chem. 7 (2005) 64-82; (g) Z. Hossaini, F.R. Charati, S. Seyfia, M. Ghambarian, Multicomponent reactions for the synthesis of functionalized 1,4-oxathiane-3-thiones under microwave irradiation in water, Chin. Chem. Lett. 24 (2013) 376-378; (h) F.R. Charati, Efficient synthesis of functionalized hydroindoles via catalystfree multicomponent reactions of ninhydrin in water, Chin. Chem. Lett. 25 (2014) 169-171. |
| [8] | J.L. Vennerstrom, M.T. Makler, C.K. Angerhofer, J.A. Williams, Antimalarial dyes revisited: xanthenes, azines, oxazines, and thiazines, Antimicrob. Agents Chemother. 39 (1995) 2671-2677. |
| [9] | R. Giri, J.R. Goodell, C.G. Xing, et al., Synthesis and cancer cell cytotoxicity of substituted xanthenes, Bioorg. Med. Chem. 18 (2000) 1456-1463. |
| [10] | K. Chibale, M. Visser, D.V. Schalkwyk, et al., Exploring the potential of xanthene derivatives as trypanothione reductase inhibitors and chloroquine potentiating agents, Tetrahedron 59 (2003) 2289-2296. |
| [11] | B.B. Bhowmik, P. Ganguly, Photophysics of xanthene dyes in surfactant solution, Spectrochim. Acta A 61 (2005) 1997-2003. |
| [12] | C.G. Knight, T. Stephens, Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles, Biochem. J. 258 (1989) 683-687. |
| [13] | M. Ahmad, T.A. King, B.H. Cha, J. Lee, Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases, J. Phys. D: Appl. Phys. 35 (2002) 1473-1476. |
| [14] |
(a) B. Das, K. Laxminarayana, M. Krishnaiah, Y. Srinivas, An efficient and convenient protocol for the synthesis of novel 12-aryl-or 12-alkyl-8,9,10,12-tetrahy-drobenzo[a]xanthen-11-one derivatives, Synlett (2007) 3107-3112; (b) J.M. Khurana, D. Magoo, pTSA-catalyzed one-pot synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones in ionic liquid and neat conditions, Tetrahedron Lett. 50 (2009) 4777-4780; (c) L. Wu, Y. Wu, F. Yan, L. Fang, HClO4-SiO2-catalyzed synthesis of 12-aryl-12Hbenzo[i][1,3]dioxolo[4,5-b]xanthene-6,11-diones and 10-aryl-6,7,8,10-tetrahy-dro-7,7-dimethyl-9H-[1,3]dioxolo[4,5-b]xanthen-9-ones, Monatsh Chem. 141 (2010) 871-875; (d) J.J. Li, W.Y. Tang, L.M. Lu, W.K. Su, Strontium triflate catalyzed one-pot condensation of β-naphthol, aldehydes and cyclic 1,3-dicarbonyl compounds, Tetrahedron Lett. 49 (2008) 7117-7120; (e) R.Z. Wang, L.F. Zhang, Z.S. Cui, Iodine-catalyzed synthesis of 12-aryl-8,9,10,12-tetrahydro-benzo[a]xanthen-11-one derivatives via multicomponent reaction, Synth. Commun. 39 (2009) 2101-2107; (f) S. Gao, C.H. Tsai, C.F. Yao, a simple and green approach for the synthesis of tetrahydrobenzo[a]-xanthen-11-one derivatives using tetrabutyl ammonium fluoride in water, Synlett (2009) 949-954; (g) J. Li, L. Lu, W. Su, A new strategy for the synthesis of benzoxanthenes catalyzed by proline triflate in water, Tetrahedron Lett. 51 (2010) 2434-2437; (h) G.C. Nandi, S. Samai, R. Kumar, M.S. Singh, An efficient one-pot synthesis of tetrahydrobenzo[a]xanthene-11-one and diazabenzo[a]anthracene-9,11-dione derivatives under solvent free condition, Tetrahedron 65 (2009) 7129-7134; (e) S. Yadav, B. Nand, J.M. Khurana, An efficient synthesis of novel 3-hydroxy-12-arylbenzo[a]xanthen-11-ones and 5,12-diarylxantheno[2,1-a]xanthene-4,12-diones using pTSA in [bmim]BF4, Can. J. Chem. 91 (2013) 698-703. |
| [15] |
(a) H. Singh, J. Sindhu, J.M. Khurana, Efficient, green and regioselective synthesis of 1,4,5-trisubstituted-1,2,3-triazoles in ionic liquid [bmim]BF4 and in taskspecific basic ionic liquid [bmim] OH, J.Iran Chem. Soc. 10 (2013) 883-888; (b) H. Singh, J. Sindhu, J.M. Khurana, Synthesis of biologically as well as industrially important 1,4,5-trisubstituted-1,2,3-triazoles using a highly efficient, green and recyclable DBU-H2O catalytic system, RSC Adv. 3 (2013) 22360-22366; (c) H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, A facile eco-friendly one-pot five-component synthesis of novel 1,2,3-triazole-linked pentasubstituted 1,4-dihydropyridines and their biological and photophysical studies, Aust. J. Chem. 66 (2013) 1088-1096; (d) J. Sindhu, H. Singh, J.M. Khurana, C. Sharma, K.R. Aneja, Multicomponent synthesis of novel 2-aryl-5-((1-aryl-1H-1,2,3-triazol-4-yl)methylthio)-1,3,4-oxa-diazoles using CuI as catalyst and their antimicrobial evaluation, Aust. J. Chem. 66 (2013) 710-717; (e) H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, Ultrasound promoted one pot synthesis of novel fluorescent triazolyl spirocyclic oxindoles using DBU based task specific ionic liquids and their antimicrobial activity, Eur. J. Med. Chem. 77 (2014) 145-154; (f) H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, Syntheses, biological evaluation and photophysical studies of novel 1,2,3-triazole linked azo dyes, RSC Adv. 4 (2014) 5915-5926.. |