Multicolor systems have gained intense interest in the past decades [1]. They have many potential applications in solar cells [2, 3],light-emitting diodes [4],and highly sensitive chemo/ biosensors [5, 6] due to their higher collective absorption [7], new photophysical properties [8],or tenability [9, 10]. Some routes have been developed to construct multicolor materials. Generally, multicolor nanoparticles or microparticles are constructed by embedding [11, 12],physical absorbing [13],or covalently bonding [14] multiple dyes or quantum dots (QDs) into particles to obtain multicolor particles. On the other hand,covalently linking dyes on polymers to produce multicolor polymers [15, 16],such as utilizing DNA as a scaffold for multiple chromophores [17]. However, embedding dyes or QDs into particles may appear aggregation of dyes in some areas,and physical absorption may be easy to make the dyes or QDs escape from the particles when the chemical environment is changed. And covalently bonding dyes into particles or on polymers need relatively complex crosslinking processes.
The self-assembly of molecular [18, 19] or macromolecular components [20, 21] into nanostructures has been gaining wide interest. For example,supramolecular self-assembly is a significant method for functionalizing materials [22, 23, 24]. Due to the inclusion complexation between numerous cyclodextrins (CDs) and functional groups on associating drug,nucleic acid,protein,and other biological substrates,CD-based supramolecular assemblies are successfully utilized in many biological fields,such as imaging and therapy [25, 26, 27].
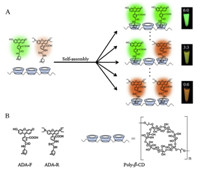
Here,a facile supramolecular self-assembly method was exploited to construct multicolour polymers,which was made up of ß-cyclodextrin polymers (poly-β-CD),adamantane (ADA)-labeled fluorescein (ADA-F) and ADA-labeled rhodamine B (ADA-R) (Fig. 1). And poly-β-CD (Mn = 94,417,PDI = 1.45) were synthesized by covalent cross-linking reaction between native β-CD and epichlorohydrin. ADA-F was synthesized from ADA and fluorescein N-hydroxysuccinimide ester,while ADA-R was synthesized from ADA and rhodamine B isothiocyanate. Utilizing the host-guest interaction between β-CD and ADA,guest molecules of ADA-F and ADA-R efficiently self-assembled on poly-β-CD with multiple CD cavities. Thus,multicolor polymers can be easily prepared by mixing different ratios of dyes with poly-β-CD. This self-assembly method for constructing multicolor polymers is one-step,facile and easily tunable.
|
Download:
|
| Fig. 1. Schematic illustration of multicolor polymers based on supramolecular selfassembly (A),and corresponding structures of adamantane-labeled luorescein (ADA-F),adamantane-labeled rhodamine B (ADA-R),and β-cyclodextrin polymers (poly-β-CD) (B). | |
Procedure of constructing multicolor polymers: For constructing multicolor polymers,6.25 mg/mL poly-β-CD were incubated with different molar ratios of ADA-F and ADA-R in PBS (pH 7.0) and vibrated for 5 h at 300 rpm and 25 ℃,respectively. The ratios of ADA-F to ADA-R were 6:0,5:1,4:2,3:3,2:4,0:6,respectively,and the total concentrations of ADA-F and ADA-R were 150 mmol/L. They were vibrated for 5 h at 300 rpm and 25 ℃. Then samples were put on agarose gel electrophoresis (3% agarose),and the electrophoresis was carried in 1× TBE at 100 V constant voltage for 20 min. Then the gel was excited by 365 nm and imaged by digital camera,and the fluorescence spectra of samples were measured by using Hitachi F-7000. Moreover,different color of polymers were separately incubated with agarose hydrogel microbeads (AHMs) and vibrated for 5 h at 1000 rpm and 25 ℃ to construct multicolor AHMs (see the details in supporting information). Two-dyes-doped (ADA-F:ADA-R = 1:1) polymers were added into buffer with different pH values from 3 to 9,and then their fluorescence spectra were measured by using Hitachi F-7000. 3. Results and discussion 3.1. The combination of dyes and poly-β-CD To confirm the combination of ADA-F or ADA-R and poly-bCD,gel electrophoresis experiments have been carried out. Under the effect of electric field,low-molecular-weight and electronegative ADA-F migrated,while ADA-F in the presence of poly-β-CD remained (Fig. 2A). Similarly,ADA-R/poly-β-CD remained,but low-molecular-weight and positively charged ADA-R migrated (Fig. 2B). These results of AGE directly suggest that ADA-F form stable ADA-F/poly-β-CD complex with poly-bCD and ADA-R form stable ADA-R/poly-β-CD complex with polyβ-CD. Furthermore,comparing with the corresponding free dyes, the fluorescence emission spectra of ADA-F/poly-β-CD and ADAR/poly-β-CD are both slightly red-shifted,and their relative fluorescence intensities increase by 1.2-fold and 7.2-fold respectively,which also indicate the formation of ADA-F/polyβ-CD and ADA-R/poly-β-CD. And the binding constant of ADA-F or ADA-R with poly-β-CD in different pH values are determined by using the Benesi-Hildebrand method (see the details in Supporting information) [29, 30]. The results indicate that the binding constants (K values) of ADA-F/poly-β-CD and ADA-R/ poly-β-CD are around 10 3 L/mol (Figs. S1 and S2 in Supporting information),which are lower than the K values of β-CD with adamantane (around 10 5 L/mol) [31] due to the increasing steric hindrance between ADA-F or ADA-R and poly-β-CD. According to the K values,above 94% of dyes have combined with β-CD cavities. Furthermore,the photostability of ADA-F/poly-β-CD and ADA-R/poly-β-CD are also investigated. After continuous illumination for 50 min,ADA-F/poly-β-CD and ADA-R/poly-β-CD show higher photostability than corresponding free dyes (Figs. S3 and S4 in Supporting information). The high brightness and high photostability make the multicolor polymers promising probes for biological imaging and bioanalytical applications.

|
Download:
|
| Fig. 2. Fluorescence emission spectra of ADA-F (λex= 490 nm) (A) and ADA-R (λex= 550 nm) (B) with or without poly-β-CD. The insert: the corresponding fluorescence images of the gel with 365 nm UV illumination. | |
In addition,in the absence of poly-β-CD,comparing the emission spectrum of mixed dyes (ADA-F:ADA-R = 1:1) with that of the same concentrations of single dyes,the emission intensity (λex= 450 nm,Fig. S8 in Supporting information) of ADA-F decrease a little and that of ADA-R do not obviously increase (Fig. 3A). While comparing the emission spectrum of two-dyedoped (ADA-F:ADA-R = 1:1) polymers with that of single-dyedoped polymers,the obviously decreased emission of ADA-F and increased emission of ADA-R demonstrates efficient FRET between ADA-F and ADA-R in the presence of poly-β-CD (Fig. 3B),and also prove successful loading of multiple dyes on one poly-β-CD. And the fluorescence excitation spectra of ADA-F/poly-β-CD overlap a part with that of ADA-R/poly-β-CD (Fig. S5 in Supporting information) also indicating that a single light source is sufficient for exciting ADA-F/poly-β-CD and ADA-R/poly-β-CD.

|
Download:
|
| Fig. 3. Fluorescence emission spectra (λex= 450 nm) of dyes in different ratios without poly-β-CD (A) and with poly-β-CD (B). The ratios indicated the ratios of ADA-F to ADA-R. | |

|
Download:
|
| Fig. 4. Multicolor polymers (A) and the corresponding gel (B) under 365 nm UV illumination. Fluorescence emission spectra (λex= 505 nm) of the multicolor polymers with different ratios of ADA-F to ADA-R(C). | |
Moreover,the integration of multicolor polymers into microbeads will not only protect dyes against different harsh chemical environments,but also providing unique optical properties to the resultant microbeads. Here,ADA-F/poly-β-CD and ADA-R/poly-bCD can separately physically diffuse and be encapsulated firmly in AHMs (Figs. S6 and S7 in Supporting information). The results are possibly attributed to the formation of multiple hydrogen bonds between poly-β-CD and AHMs. And easy-to-discern colors of AHMs from green to orange are also observed (Fig. S8 in Supporting information). Thus,it is a convenient and facile method for constructing multicolor microbeads by embedding multicolor polymers. We envision that further optimization of this method will allow multiplexed screening assays of the hybrid hydrogel microbeads technology.
|
Download:
|
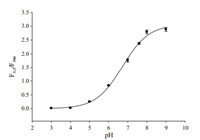
| Fig. 5. Plotting F525/F590 of the two-dyes-doped (ADA-F:ADA-R = 1:2) polymers versus pH values. F525and F590indicated the fluorescence intensity of ADA-F at 525 nm and ADA-R at 590 nm (λex= 505 nm) in polymers,respectively | |
In addition,as pH-sensitive fluorescein and pH insensitive rhodamine B are usually used as ratiometric fluorescent probes for pH sensing [32],so the fluorescence intensities of two-dye-doped polymers (ADA-F:ADA-R = 1:2) vary with the change of pH values (Fig. S9 in Supporting information). Interestingly,the relative ratios of fluorescence intensity of ADA-F at 525 nm to that of ADAR at 590 nm (F525/F590) increase by 161-fold (from 0.018 to 2.9) over the pH range of 3-9 (Fig. 5),which covers most of the pH ranges for biological applications (for example,intracellular pH measurement). Thus,the two-dye-doped polymers also have the potential as ratiometric fluorescent probes for pH sensing. 4. Conclusion In summary,here we have described a one-step and facile selfassembly approach for constructing multicolor polymers. And the advantages of multicolor polymers,such as easy tunablity,intense fluorescence,high photostability,make these polymers promising probes for biological imaging and bioanalytical applications. Currently,intensive research using the two-dye-doped polymers for intracellular pH measurement is being conducted in our lab. Hence,this facile self-assembly method should be enough for the foreseeable future of constructing functionalized materials for bioanalyses and imaging.
Acknowledgments This study was supported by the National Natural Science Foundation of China (Nos. 21190044,21175035),National Basic Research Program (No. 2011CB911002),International Science & Technology operation Program of China (No. 2010DFB30300). Appendix A. Supplementary data Supplementary material related to this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet.2014. 05.051.| [1] | E. Schwartz, S. Le Gac, J.J. Cornelissen, et al., Macromolecular multi-chromophoric scaffolding, Chem. Soc. Rev. 39 (2010) 1576-1599. |
| [2] | E.C. Greyson, B.R. Stepp, X. Chen, et al., Singlet exciton fission for solar cell applications: energy aspects of interchromophore coupling, J. Phys. Chem. B 114 (2009) 14223-14232. |
| [3] | B.E. Hardin, E.T. Hoke, P.B. Armstrong, et al., Increased light harvesting in dyesensitized solar cells with energy relay dyes, Nat. Photon. 3 (2009) 406-411. |
| [4] | A.C. Grimsdale, K. Leok Chan, R.E. Martin, et al., Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices, Chem. Rev. 109 (2009) 897-1091. |
| [5] | M. Shi, J. Chen, Y. Huang, et al., A multicolor nano-immunosensor for the detection of multiple targets, RSC Adv. 3 (2013) 13884-13890. |
| [6] | J.Z. Song, Q. Yang, F.T. Lv, L.B. Liu, S. Wang, Visual detection of DNA mutation using multicolor fluorescent coding, ACS Appl. Mater. Interfaces 4 (2012) 2885-2890. |
| [7] | S. Günes, H. Neugebauer, N.S. Sariciftci, Conjugated polymer-based organic solar cells, Chem. Rev. 107 (2007) 1324-1338. |
| [8] | F.J. Hoeben, P. Jonkheijm, E. Meijer, P.H.J. Schenning Albertus, About supramolecular assemblies of π-conjugated systems, Chem. Rev. 105 (2005) 1491-1546. |
| [9] | A. Datta, S.K. Pati, Dipolar interactions and hydrogen bonding in supramolecular aggregates: understanding cooperative phenomena for 1st hyperpolarizability, Chem. Soc. Rev. 35 (2006) 1305-1323. |
| [10] | G.C. Bazan, Novel organic materials through control of multichromophore interactions, J. Org. Chem. 72 (2007) 8615-8635. |
| [11] | E. Herz, A. Burns, D. Bonner, et al., Large stokes-shift fluorescent silica nanoparticles with enhanced emission over free dye for single excitation multiplexing, macro, Rapid Commun. 30 (2009) 1907-1910. |
| [12] | L. Wang, W.H. Tan, Multicolor FRET silica nanoparticles by single wavelength excitation, Nano. Lett. 6 (2006) 84-88. |
| [13] | C.N. Allen, N. Lequeux, C. Chassenieux, et al., Optical analysis of beads encoded with quantum dots coated with a cationic polymer, Adv. Mater. 19 (2007) 4420-4425. |
| [14] | J.B. Liu, X. Yang, K.M. Wang, et al., Combining physical embedding and covalent bonding for stable encapsulation of quantum dots into agarose hydrogels, J. Mater. Chem. 22 (2012) 495-501. |
| [15] | H. Nishi, T. Namari, S. Kobatake, Photochromic polymers bearing various diarylethene chromophores as the pendant: synthesis, optical properties, and multicolor photochromism, J. Mater. Chem. 21 (2011) 17249-17258. |
| [16] | J.N. Wilson, E.T. Kool, Fluorescent DNA base replacements: reporters and sensors for biological systems, Org. Biomol. Chem. 4 (2006) 4265-4274. |
| [17] | Y.N. Teo, E.T. Kool, DNA-multichromophore systems, Chem. Rev. 112 (2012) 4221-4245. |
| [18] | W.Y. Yuan, Z.S. Lu, C.M. Li, Self-assembling microsized materials to fabricate multifunctional hierarchical nanostructures on macroscale substrates, J. Mater. Chem. A 1 (2013) 6416-6424. |
| [19] | M. Vendrell, D. Zhai, J.C. Er, Y.T. Chang, Combinatorial strategies in fluorescent probe development, Chem. Rev. 112 (2012) 4391-4420. |
| [20] | T. Tsuruoka, H. Kawasaki, H. Nawafune, et al., Controlled self-assembly of metalorganic frameworks on metal nanoparticles for efficient synthesis of hybrid nanostructures, ACS Appl. Mater. Interfaces 3 (2011) 3788-3791. |
| [21] | O.I. Wilner, I. Willner, Functionalized DNA nanostructures, Chem. Rev. 112 (2012) 2528-2556. |
| [22] | B. Mu, Y.R. Kang, A.Q. Wang, Preparation of a polyelectrolyte-coated magnetic attapulgite composite for the adsorption of precious metals, J. Mater. Chem. A 1 (2013) 4804-4811. |
| [23] | E. Busseron, Y. Ruff, E. Moulin, N. Giuseppone, Supramolecular self-assemblies as functional nanomaterials, Nanoscale 5 (2013) 7098-7140. |
| [24] | W. Tao, Y. Liu, B. Jiang, et al., A linear-hyperbranched supramolecular amphiphile and its self-assembly into vesicles with great ductility, J. Am. Chem. Soc. 134 (2012) 762-764. |
| [25] | E. Deniz, N. Kandoth, A. Fraix, et al., Photoinduced fluorescence activation and nitric oxide release with biocompatible polymer nanoparticles, Chem. Eur. J. 18 (2012) 15782-15787. |
| [26] | L.M. Chen, X. Zhao, Y. Lin, et al., A supramolecular strategy to assemble multifunctional viral nanoparticles, Chem. Commun. 49 (2013) 9678-9680. |
| [27] | L. Wang, L.L. Li, H.L. Ma, et al., Recent advances in biocompatible supramolecular assemblies for biomolecular detection and delivery, Chin. Chem. Lett. 24 (2013) 351-358. |
| [28] | C. Koopmans, H. Ritter, Formation of physical hydrogels via host-guest interactions of β-cyclodextrin polymers and copolymers bearing adamantyl groups, Macromolecules 41 (2008) 7418-7422. |
| [29] | H.A. Benesi, J.H. Hildebrand, A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons, J. Am. Chem. Soc. 71 (1949) 2703-2707. |
| [30] | V. Indirapriyadharshini, P. Karunanithi, P. Ramamurthy, Inclusion of resorcinolbased acridinedione dyes in cyclodextrins: fluorescence enhancement, Langmuir 17 (2001) 4056-4060. |
| [31] | G.S. Chen, M. Jiang, Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly, Chem. Soc. Rev. 40 (2011) 2254-2266. |
| [32] | W. Shi, X.H. Li, H.M. Ma, A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intracellular pH of whole cells, Angew. Chem. Int. Ed. 51 (2012) 6432-6435. |




